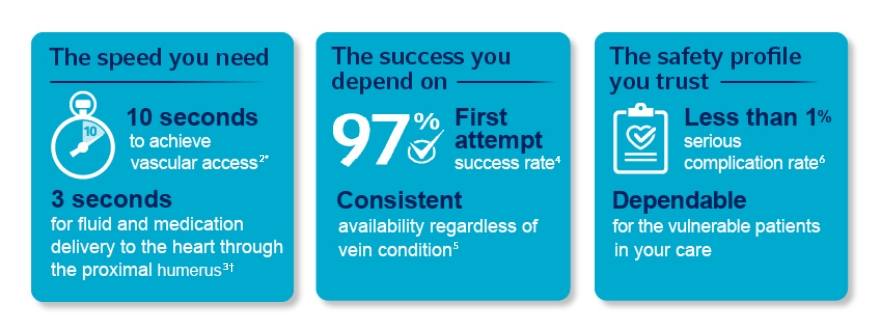
Arrow™ EZ-IO™ Intraosseous
Vascular Access System:
Vascular Access System:
EZ-Stabilizer™ Dressing:
Secure catheter placement
Secure catheter placement
- Protects the insertion site.
- Recommend for use with all EZ-IO™ Needle Set insertions.
NeedleVISE™ Sharps Block:
- Promotes sharps safety.
Arrow™ EZ-Connect™ Extension Set:
- Reinforced 90º angle to help prevent your line from kinking.
Arrow™ EZ-IO™ Power Driver:
Ready-to-use when every moment matters
Ready-to-use when every moment matters
- No charging necessary; no downtime or additional equipment needed.
- Designed for use in ground and air transport, hospital, and military environments.
- Fully sealed design for quick and easy cleaning.
- Green/red battery indicator light.
Arrow™ EZ-IO™ Needle Set:
Supporting your success under pressure
Supporting your success under pressure
- The only IO system FDA cleared for up to 48-hour dwell.**
- Color-coded needle system enables quick selection and post-insertion identification.
- Diamond needle tip designed for precision performance.
- One needle with two insertion options allows for powered or manual (if necessary) insertion.