Arrow® EZ-IO® Intraosseous Vascular Access System
When every moment counts, The Arrow EZ-IO System allows clinicians to gain fast, effective vascular access through the intraosseous space without many serious complications associated with central venuous catheters.
REQUEST A DEMO





|

|

|

|
|---|---|---|---|
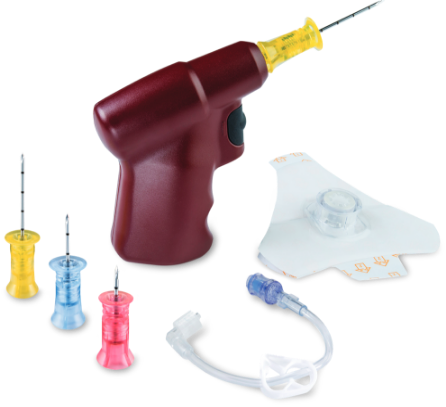
| EZ-IO Needles |
EZ-IO Vascular Access Driver |
EZ-Stabilizer Dressing |
EZ- Connect Extension Set |
|
Diamond needle tip designed for precision performance and effective manual insertion if needed. |
Enables clinician control of the insertion with proven tactile feedback. |
Designed to provide flexible securement of the EZ-IO Catheter. |
Must be primed with 1 mL of fluid prior to use. |
Arrowg+ard Blue®
MAC
Multi-lumen Access Catheter
The Arrowg+ard Blue® MAC is impregnated with antimicrobial protection along the extraluminal surface of the catheter body. This protects against gram positive and gram-negative bacteria, fungi and yeast.1 The multi-lumen access catheter meets the highest level of recommendation by the CDC (Recommendation 1A), INS (Level 1) and SHEA (Level 1). 2-5
REQUEST A DEMO





|

|

|
|---|---|---|
|
High flow rates of up to 29 liters per hour under gravity alone. |
The catheter combines the access of a sheath introfucer with the high-flow lumens of a central line. It also includes companion catheters that add up to three lumens. |
Meets a variety of patient types: Septic shock, Trauma, Cardiac surgery, Transplant surgery, and High risk for cather-related bloodstream infections (CRBSI) |

QuikClot®
Hemostatic Dressings
Safe and intuitive as standard gauze,7 the QuikClot portfolio of products can handle a variety of bleeding scenarios.8 When you don't know what bleeding scenario will be wheeled through that door, it's QuikClot or it's not.
REQUEST A DEMO




|

|

|
|---|---|---|
|
Faster time to hemostasis9,10 |
Robust clot formation11,12 |
Fewer rebleeds11,13 |
Rusch® and Sheridan®
Endotracheal Tubes
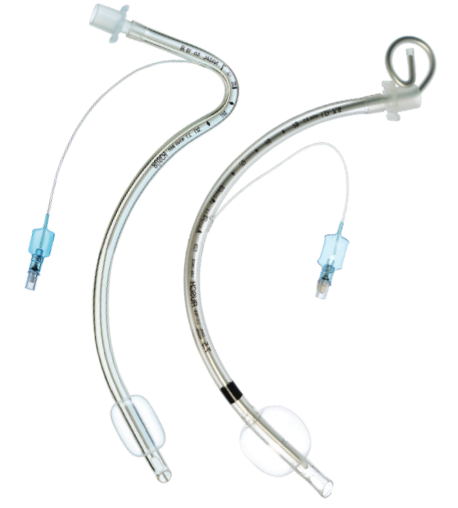
All endotracheal tubes are designed to feature and excellent I.D. to O.D. ratio, biocompatible materials, thin-walled low-pressure cuff design and a smooth tip to facilitate safe insertion.
REQUEST A DEMO



|

|
|---|---|
|
We offer endotracheal tubes for short or long-term intubation, from high to low-volume, cuffed or uncuffed, so you have the right airway device for every patient. |
+ Single-use + Sterile + Not made with natural rubber latex |