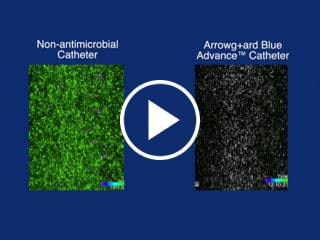
- Proven effective to reduce catheter colonization by major CLABSI-causing pathogens for at least 29 days1
- Proven to reduce intraluminal and extraluminal fibrin sheath accumulation for at least 29 days2,3
- Proven reduction of thrombotic occlusion within catheter for at least 29 days3
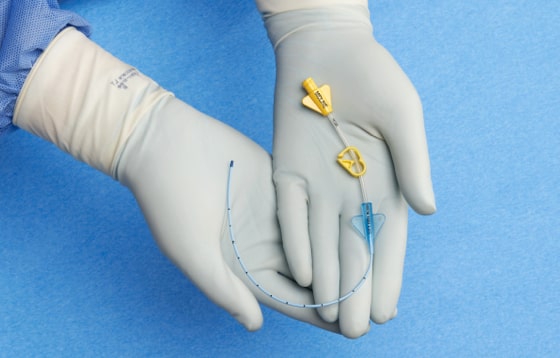
- Brightly colored yellow hubs and bold labeling facilitate easy midline identification
- Additional midline catheter identification sticker and overbed sign, for extra awareness
- Infuse with confidence!
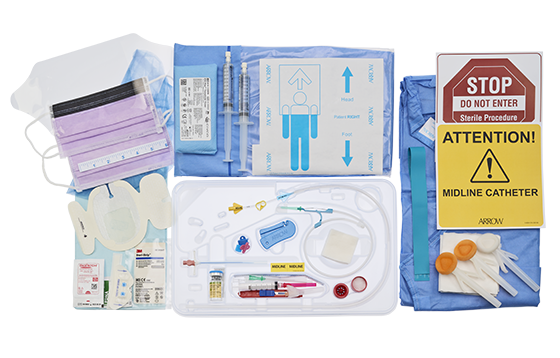
All-inclusive ergonomic Arrow® Midline kits provide everything you need for bedside insertion including pre-packaged components for less prep time. Additional packaging options available to make it easy to find the right kit to fit your needs.
- Redesigned Arrow® Trimmer with visualization window
- Ultrasound probe cover with gel
- Prefilled Saline Syringes packaged inside the kit
- Available with 3M™ Tegaderm™ CHG Dressing or BioPatch® Protective Disk
- Access Teleflex Academy for free educational offerings with in-person and virtual programs geared to teach insertion techniques based on the CDC, INS, and SHEA guidelines including best practices, standards of care, and vessel health
- Side-by-side clinical support with Teleflex experts when you need it
Product Features
Pressure injection
Up to 5mL/second through the distal lumen
Up to 5mL/second through the distal lumen
Single and dual lumen design options available
Arrow® TaperFree™ Catheter
Meets INS 45% Catheter to Vein Ratio recommendations6
Meets INS 45% Catheter to Vein Ratio recommendations6
Antimicrobial/antithrombogenic
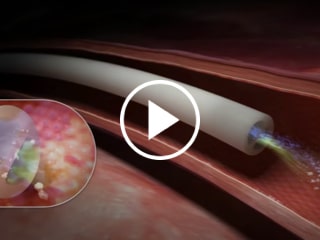
Chlorhexidine-based technology provides protection on the external catheter surface from tip to juncture hub and the entire intraluminal pathway including the luer hub and extension lines1,2
Chlorhexidine-based technology provides protection on the external catheter surface from tip to juncture hub and the entire intraluminal pathway including the luer hub and extension lines1,2
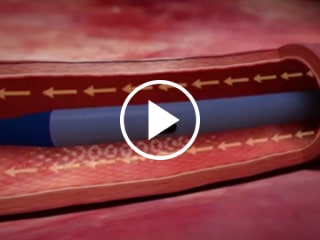
Staggered exit ports
Reduces risk of mixing incompatible drugs and solutions that may create precipitate4
Reduces risk of mixing incompatible drugs and solutions that may create precipitate4
Blue FlexTip® Catheter
Proprietary design minimizes risk of vessel damage5
Proprietary design minimizes risk of vessel damage5
Bright yellow pinch clamps and hubs for easy midline identification.
See the Arrow® Midline Insertion Procedure
Watch It Now
Additional Resources
Blue FlexTip® Feature vs. Trimmed
Catheter
Staggered vs Adjacent Exit Ports Catheter
Arrowg+ard Blue Advance® Protection - Biofilm Formation
TaperFree™ vs. Rev. Taper Catheter
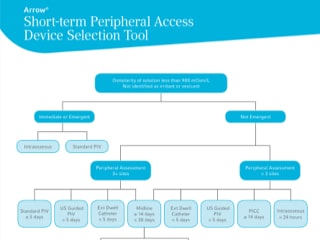
Arrow® Short-term Peripheral Access Device Selection Tool