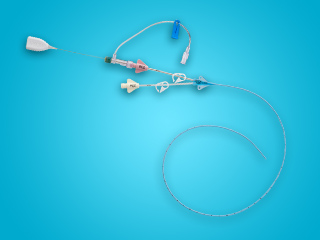
Extraluminal and intraluminal protection help reduce colonization from pathogens responsible for most healthcare-associated infections6

- Reduces thrombus accumulation on catheter surfaces5
- Features chlorhexidine that works continuously to prevent catheter buildup of fibrin sheath, reducing the risk of intraluminal thrombotic occlusion7
In vivo intravascular studies have shown an average of 72% less intimal hyperplasia after 30 days and a reduction in phlebitis5
To help provide the tools that are specific to clinicians’ needs, Arrow™ PICCs are available in a range of different kits, including:
- Max Barrier Kits
- Interventional Radiology Kits
- Custom PICC Kits
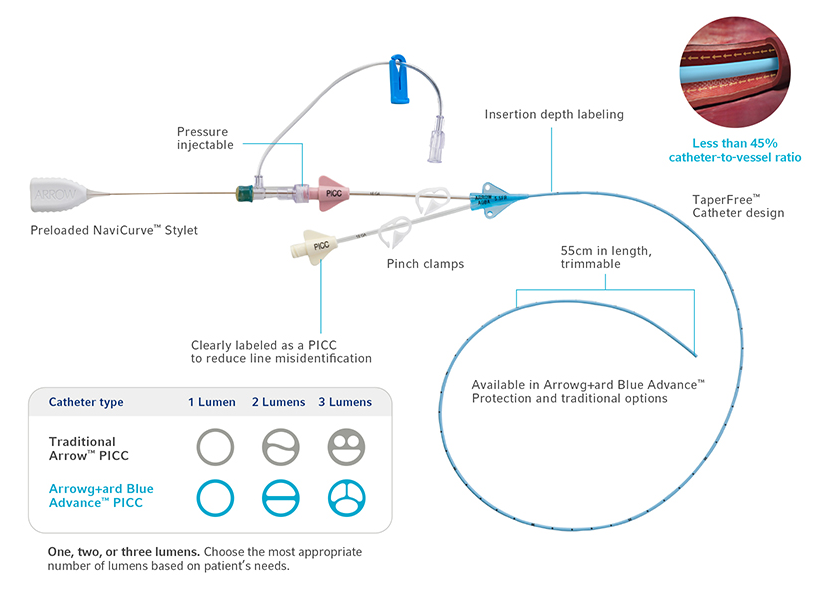
Kit Features
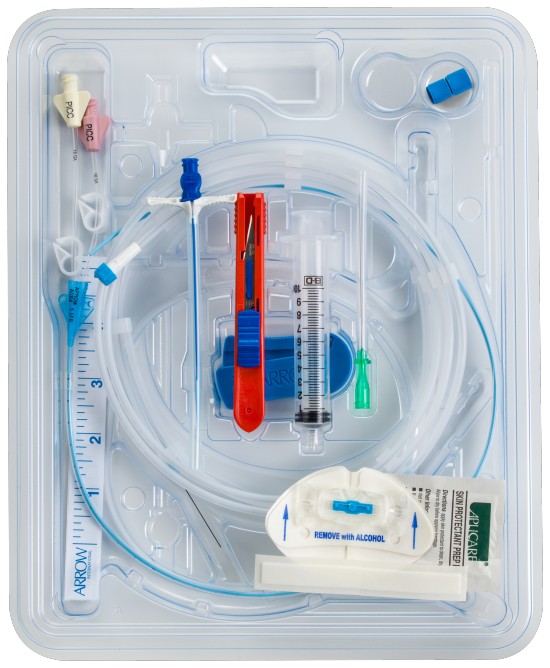
All-inclusive ergonomic Arrow™ Max Barrier PICC Kits are designed to provide everything you need for bedside insertion, including pre-packaged components for less prep time
- One-piece drape
- Redesigned Arrow™ Trimmer with visualization window
- Ultrasound probe cover with gel
- Prefilled Saline Syringes packaged inside the kit
- Available with 3M™ Tegaderm™ CHG I.V. Securement Dressing or BioPatch® Protective Disk
- Extra ChloraPrep® Skin Prep applicators in each kit
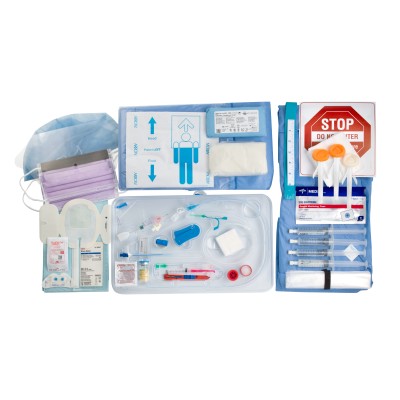
Kit Features
- Arrow™ Traditional and Arrowg+ard Blue Advance™ PICC kits are designed specifically for your IR needs
- Simplified kit with single-tray design
- No need to open full kit to complete procedure