Customer Support
South Africa Office Address
Teleflex Medical (Pty) Ltd. 1st Floor, Block C, Stoneridge Office Park, 8 Greenstone Place, Greenstone, Edenvale, 1609, Johannesburg, South Africa Phone: +27(0)11 807 4887 Fax: +27 (0)11 807 4994
South Africa Mail Address
Teleflex Medical (Pty) Ltd. P.O. Box 6882, Greenstone, 1616
Email Teleflex Medical Ltd.MANTA Vascular Closure Device
The MANTA Device is the first commercially available biomechanical vascular closure device designed specifically for large bore femoral arterial access site closure.1 Available in 14 Fr. and 18 Fr., a single MANTA Device effectively closes femoral arterial access sites following the use of sheaths ranging from 12 Fr. to 25 Fr. O.D.2a
Simple Deployment
Addresses the challenges of large bore closure with a single easy-to-use
device.2a
Rapid Hemostasis
Reduces time to hemostasis without pre-closure, utilizing the
coagulation-inducing properties of collagen for rapid hemostasis to promote
vessel healing.2c,3-5
Reliable Closure
Delivers reproducible results and helps inspire confidence in achieving
successful closure.2b

Clinically Proven
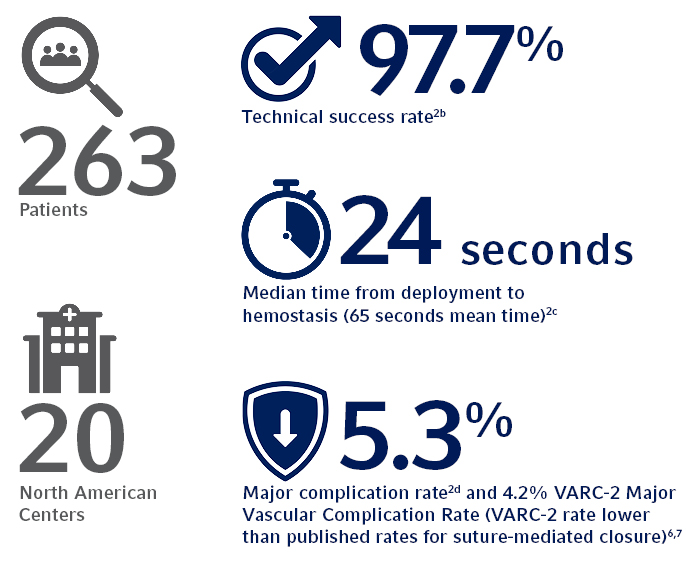
The SAFE MANTA IDE Clinical Trial, the largest U.S. prospective, multi-center study
of a purpose-designed large bore femoral arterial access site closure device to
date, demonstrated the safety and effectiveness of the MANTA Device
with all primary and secondary endpoints met.2
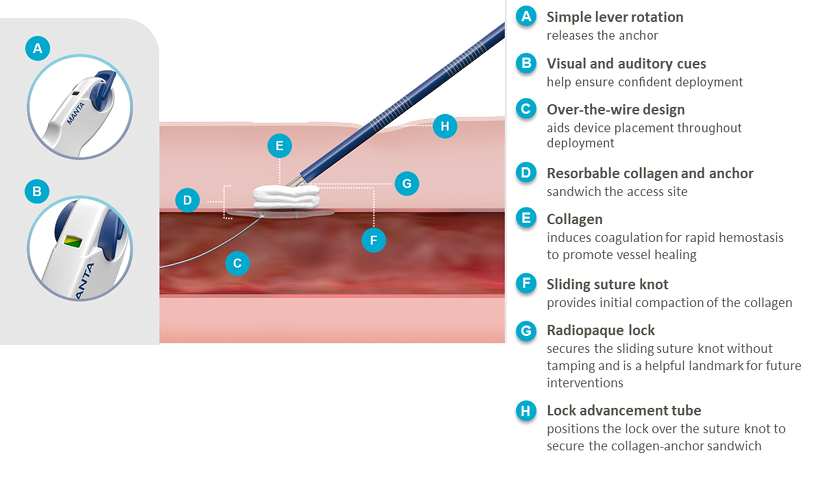
Product Features
Learn More About the MANTA Device
References:
- Data on file at Teleflex.
- Data on file at Teleflex. The SAFE MANTA IDE Clinical Trial.
a. A single MANTA Vascular Closure Device was deployed in 99.6% of subjects in IDE trial.
b. 97.7% Technical Success, defined as percutaneous vascular closure obtained with the MANTA < Device without the use of unplanned endovascular or surgical intervention.
c. The MANTA Device demonstrated a time to hemostasis (TTH) of 24 seconds median time (65 seconds mean time) from deployment to hemostasis, which is lower than published rates for Perclose ProGlide® where Perclose ProGlide® demonstrated a TTH of 9.8 +/- 17 minutes (588 +/- 1,020 seconds).3
d. Major Complications defined as composite of: i) vascular injury requiring surgical repair/stent-graft; ii) bleeding requiring transfusion; iii) lower extremity ischemia requiring surgical repair/additional percutaneous intervention; iv) nerve injury (permanent or requiring surgical repair); and v) infection requiring IV antibiotics and/or extended hospitalization.
Study sponsored by Teleflex Incorporated or its affiliates. - Nelson PR, et al. A multicenter, randomized, controlled trial of totally percutaneous access versus open femoral exposure for endovascular aortic aneurysm repair (the PEVAR trial). J Vasc Surg. 2014 May;59(5):1081-1193.
- Farndale RW, Sixma JJ, Barnes MJ, de Groot, PG. The role of collagen in thrombosis and hemostasis, J Thromb Haemost. 2004 Apr,2(4);564-573.
- Nuyttens BP, Thijs T, Deckmyn H, Broos K. Platelet adhesion to collagen, Thromb Res. 2011;127(2); S26-S29.
- Généreux P, et al. Vascular complications after transcatheter aortic valve replacement. J Am Coll Cardiol. 2012 Sept 18;60(12):1043-1052.
- Lauten A, et al. Percutaneous left-ventricular support with the Impella 2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circ Heart Fail. 2013 Jan;6(1):23-30.