Latin America /
Product Areas /
Vascular Access /
Central Access /
Short-Term Central Venous Catheters (CVC)
Arrowg+ard Blue Plus® CVC
Help protect your patients from catheter-related complications.
The Arrowg+ard Blue Plus® CVC is the only full-spectrum antimicrobial CVC that protects against:
- Gram-positive bacteria
- Gram-negative bacteria
- Fungi
Arrowg+ard Blue Plus® Protection is chemically bonded to the catheter, providing antimicrobial protection on the catheter surface.
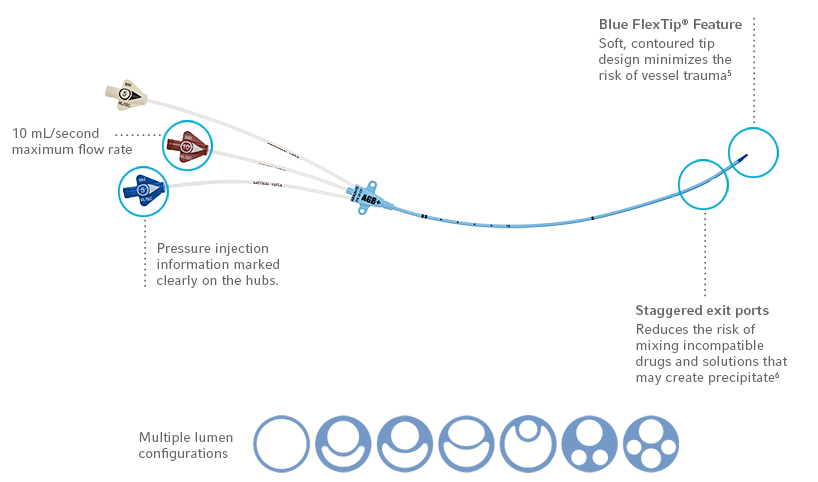
- Complete coverage – extension lines, hub and catheter protected1
- In vitro performance – proven effectiveness against common antibiotic resistant “super bugs”2
Meets Guidelines and Recommendations:
- Centers for Disease Control (CDC)*3
- The Society for Healthcare Epidemiology of America (SHEA)**4

References:
- https://www.accessdata.fda.gov/cdrh_docs/pdf/K993691.pdf
- Gupta N, Weber H, Moss S, Gaire-Patel K. Are antibiotic resistant “super bugs” a real challenge to antimicrobial central venous catheter performance? AVA 2014.
- O’Grady NP, Alexander M, Burns LA, Dellinger P, Garland J, Heard SO et al Guidelines for the Prevention of Intravascular Catheter-related Infections, 2011. The Centers for Disease Control. www.cdc.gov/hicpac/pdf/guidelines/bsi-guidelines-2011.pdf
- Buetti, N., Marschall, J., Drees, M., Fakih, M., Hadaway, L., Maragakis, L., . . . Mermel, L. (2022). Strategies to prevent central line-associated bloodstream infections in acute-care hospitals: 2022 Update. Infection Control & Hospital Epidemiology, 1-17. doi:10.1017/ice.2022.87
- Rosenbauer KA, Herzer JA. Surface morphology and tensile force at breaking point or different kinds of intravenous catheters before and after usage. Scan Electron Microsc. 1981;(Pt 3):125-30.
- Collins JL, Lutz RJ. In vitro Study of Simultaneous Infusion of Incompatible Drugs in Multilumen Catheters. Heart & Lung. 1991; 20(3):271-7.
*Use a chlorhexidine/silver sulfadiazine or minocycline/rifampin -impregnated
CVC in patients whose catheter is expected to remain in place >5 days if, after successful
implementation of a comprehensive strategy to reduce rates of CLABSI, the CLABSI rate is not
decreasing.
**Use such catheters in the following instances: i. Hospital units or patient populations have a CLABSI rate above institutional goals despite compliance with basic CLABSI prevention practices. Some evidence suggests that use of antimicrobial CVCs may have no additional benefit in patient care units that have already established a low incidence of catheter infections. ii. Patients have limited venous access and a history of recurrent CLABSI. iii. Patients are at heightened risk of severe sequelae from a CLABSI (e.g, patients with recently implanted intravascular devices, such as a prosthetic heart valve or aortic graft).
Contraindication:
The Arrowg+ard Blue Plus® CVC is contraindicated for patients with known hypersensitivity to chlorhexidine and silver sulfadiazine and/or sulfa drugs.
Federal Law (USA) restricts these devices to sale by or on the order of a physician.
Teleflex, the Teleflex logo, and Arrow, Arrowg+ard Blue Plus, and Blue FlexTip are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. All other trademarks are the trademarks of their respective owners. Please check your local regulatory approval status. Refer to the applicable Instructions for Use for the indications approved in your geography.
Information in this material is not a substitute for the product Instructions for Use. This document does not imply compatibility between devices. Not all products may be available in all countries. Please contact your local representative. Revised: 08/2022. ©2022 Teleflex Incorporated. All rights reserved. MC-007978 LA EN
**Use such catheters in the following instances: i. Hospital units or patient populations have a CLABSI rate above institutional goals despite compliance with basic CLABSI prevention practices. Some evidence suggests that use of antimicrobial CVCs may have no additional benefit in patient care units that have already established a low incidence of catheter infections. ii. Patients have limited venous access and a history of recurrent CLABSI. iii. Patients are at heightened risk of severe sequelae from a CLABSI (e.g, patients with recently implanted intravascular devices, such as a prosthetic heart valve or aortic graft).
Contraindication:
The Arrowg+ard Blue Plus® CVC is contraindicated for patients with known hypersensitivity to chlorhexidine and silver sulfadiazine and/or sulfa drugs.
Federal Law (USA) restricts these devices to sale by or on the order of a physician.
Teleflex, the Teleflex logo, and Arrow, Arrowg+ard Blue Plus, and Blue FlexTip are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. All other trademarks are the trademarks of their respective owners. Please check your local regulatory approval status. Refer to the applicable Instructions for Use for the indications approved in your geography.
Information in this material is not a substitute for the product Instructions for Use. This document does not imply compatibility between devices. Not all products may be available in all countries. Please contact your local representative. Revised: 08/2022. ©2022 Teleflex Incorporated. All rights reserved. MC-007978 LA EN