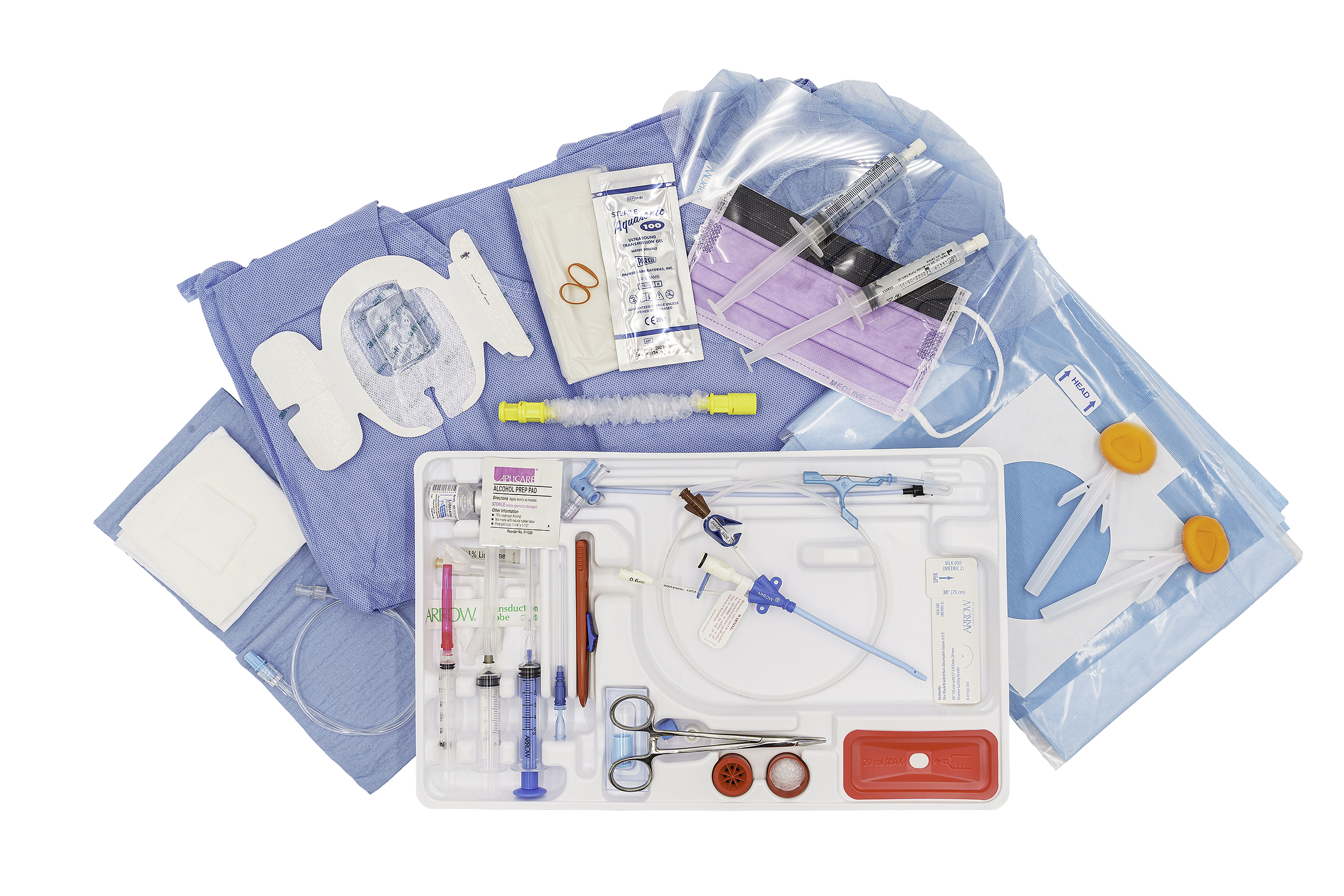
-
Pressure Injectable VANTEX Central Venous Catheters Indications For Use. Williamston,
MI: Centurion Medical Products; 4.
-
Gupta N, Weber H, Moss S, Gaire-Patel K. Are antibiotic resistant “super bugs”
a real challenge to antimicrobial central venous catheter performance? AVA 2014.
-
O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the Prevention of Intravascular
Catheter-Related Infections, 2011 (Revised 2017). 2. Atlanta, GA: Centers for Disease
Control and Prevention; 2017.
-
Buetti, N., Marschall, J., Drees, M., Fakih, M., Hadaway, L., Maragakis, L., . . . Mermel,
L. (2022). Strategies to
prevent central line-associated bloodstream infections in acute-care hospitals: 2022 Update.
Infection Control &
Hospital Epidemiology, 1-17. doi:10.1017/ice.2022.87
-
Gorski L, Hadaway L, Hagle ME, McGoldrick M, et al. Infusion Therapy Standards of Practice.
Journal of Infusion Nursing. 2016; Jan 39(1S).
-
Occupational Safety & Health Administration Regulations (Standards – 29 CFR). Part
1910.1030: Bloodborne pathogens. Occupational Safety & Health Administration Web site.
https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030. Accessed on
February 12, 2020.