Emergency Room
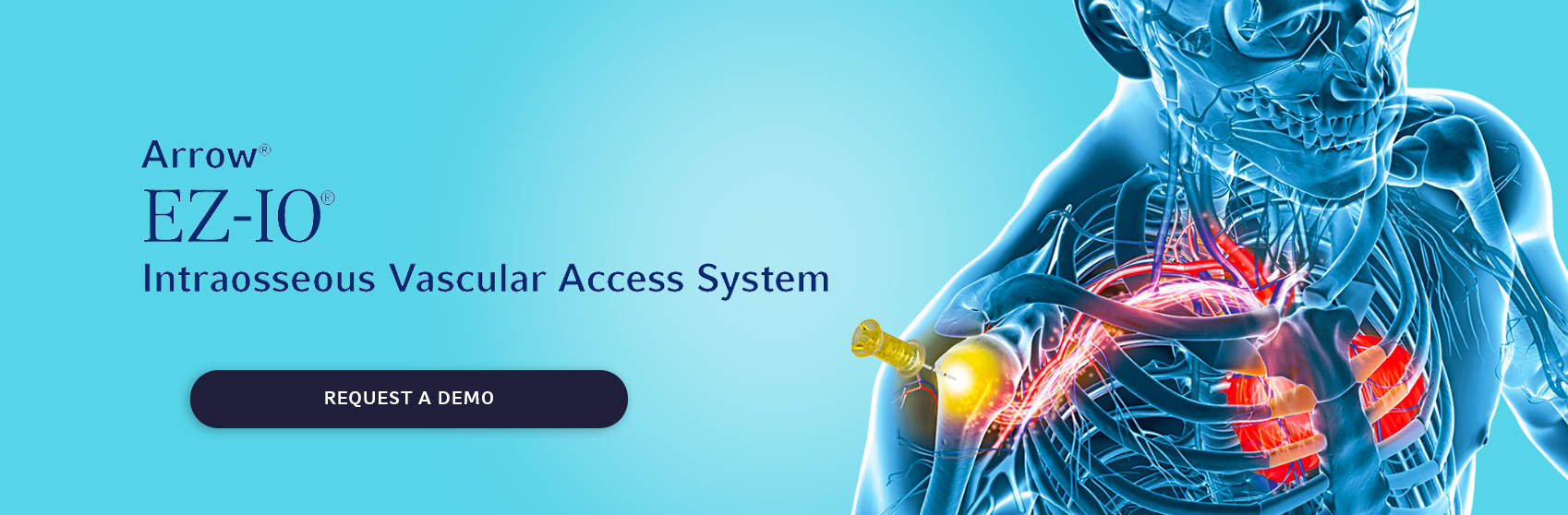
Arrow® EZ-IO®
Intraosseous Vascular Access System

In any situation where intravenous access is difficult to obtain in emergent, urgent, or medically necessary cases, the Arrow® EZ-IO® Intraosseous Vascular Access System from Teleflex is a proven,1 fast2*, and effective3 solution.
The system is indicated for up to 24 hours and may be extended for up to 48 hours for patients ≥ 12 years old when alternate intravenous access is not available or reliably established. The EZ-IO® System provides peripheral venous access with central venous catheter performance.4-5*†
REQUEST A DEMO

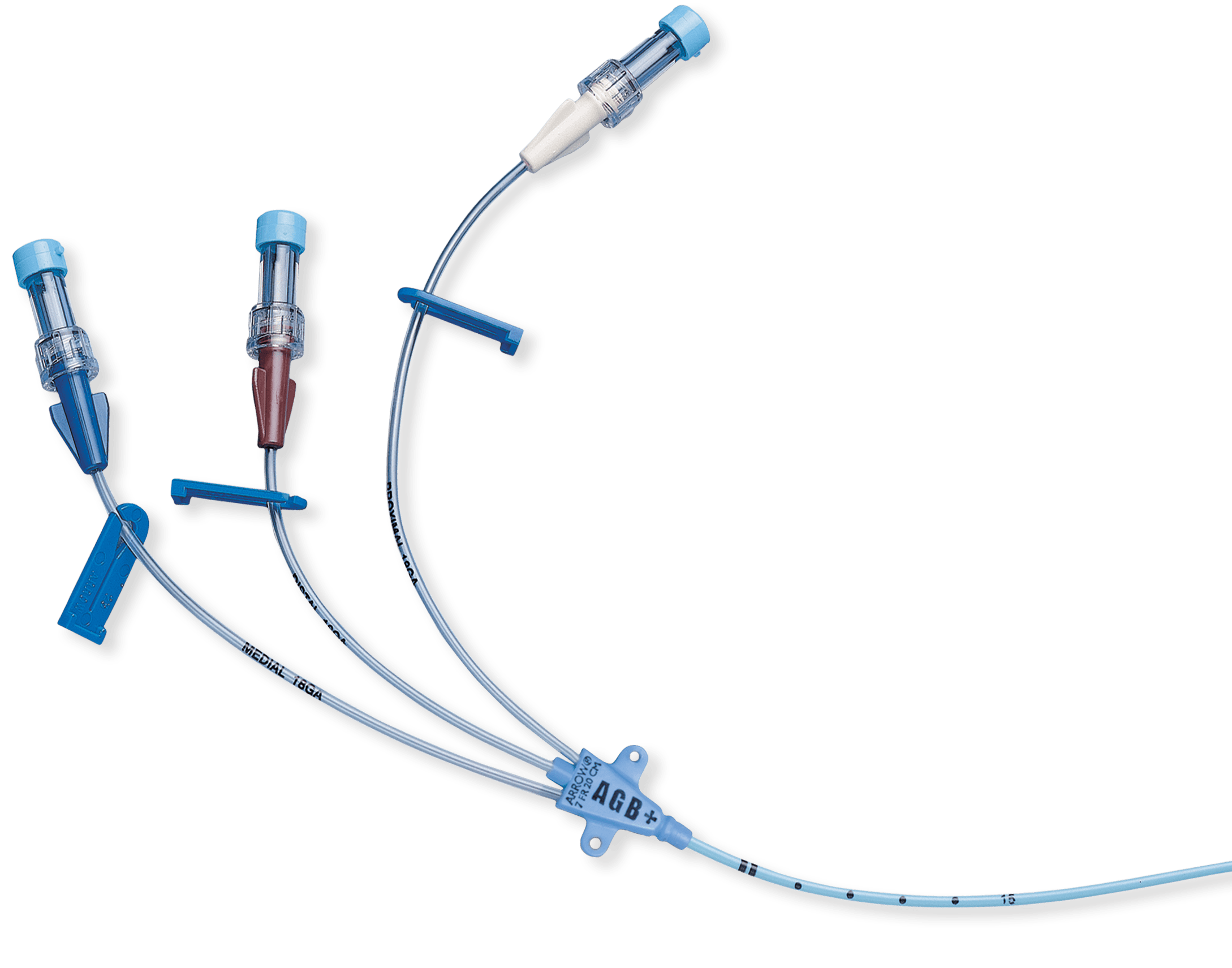
Arrow®
Arrowg+ard Blue Plus®
Central Venous Catheters

The Arrowg+ard Blue Plus® CVC is the only full-spectrum antimicrobial CVC that protects against gram-positive bacteria, gram-negative bacteria, & fungi
Arrowg+ard Blue Plus® Protection is chemically bonded to the catheter, providing antimicrobial protection on the catheter surface:
- Complete coverage – extension lines, hub and catheter protected6.
- In vitro performance – proven effectiveness against common antibiotic resistant “super bugs”7
Meets Guidelines and Recommendations:
- Center for Disease Control (CDC)*8
- The Society for Healthcare Epidemiology of America (SHEA)**9
REQUEST A DEMO
Rusch® Single-Use
Laryngoscopes


Rüsch® Single-Use Laryngoscopes offer the weight and feel of a reusable blade with the convenience of a single-use blade. Rüsch® Standard Single-Use Blades also eliminate the hassles of reusable blades, including the time and cost of reprocessing, without sacrificing strength or light quality.
REQUEST A DEMO



Rusch® DisposeLED® Handle

- LED lamp provides optimal light output
- Ergonomic design for optimized grip
- Batteries included and easy to remove after use
- Individually packaged for single-patient use
- Light can be tested in package without opening
REQUEST A DEMO
Rusch® and Sheridan®
Endotracheal Tubes
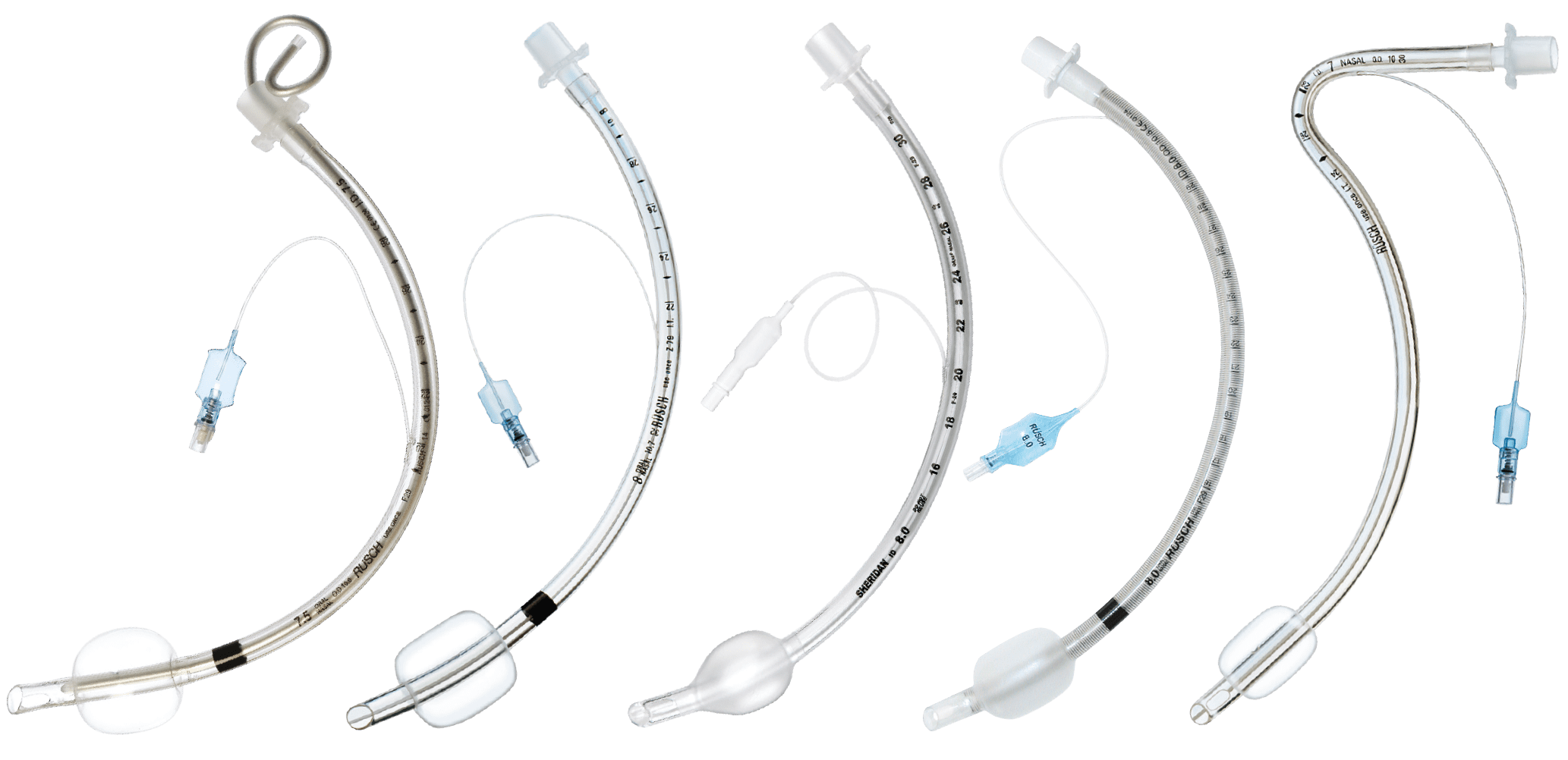
Thanks to an extensive and versatile range of Rüsch® and Sheridan® Endotracheal Tubes, Teleflex delivers a quality solution for both you and your patient.
All endotracheal tubes are designed to feature an excellent I.D. to O.D. ratio, biocompatible materials, thin-walled low-pressure cuff design and a smooth tip to facilitate safe insertion.
REQUEST A DEMO