Customer Support
IDA Business and Technology Park
Dublin Road, Athlone
Co Westmeath
Ireland
Phone: +353 (0) 9 06 46 08 00
Fax: +353 (0) 14 37 07 73
Email Europe, Middle East, Africa Regional
Office
United Kingdom
Teleflex
Oakfield House
59 Hill Avenue
Amersham
HP6 5BX
Phone: +44 (0)1494 53 27 61
Fax: +44 (0)1494 52 46 50
Email Teleflex Medical
South Africa Office Address
Teleflex Medical (Pty) Ltd.
1st Floor, Block C,
Stoneridge Office Park,
8 Greenstone Place,
Greenstone, Edenvale, 1609,
Johannesburg, South Africa
Phone: +27(0)11 807 4887
Fax: +27 (0)11 807 4994
South Africa Mail Address
Teleflex Medical (Pty) Ltd.
P.O. Box 6882, Greenstone, 1616
Email Teleflex Medical Ltd.
Passeo™-18 Lux™ DCB
Clinically proven results in challenging patient groups
The Passeo™-18 Lux™ Drug-Coated Balloon is indicated to dilate de novo or restenotic lesions in the infrainguinal arteries* through a low profile** balloon platform. Its technologies allow an effective drug delivery1: The SafeGuard™ Insertion Aid reduces drug loss in the introducer sheath up to 94% and protects user and balloon from contact and damage, while the Lux™ Coating (Paclitaxel and BTHC) optimizes drug transfer and ensures high drug retention at the lesion site.
Product Highlights

Clinically Proven
Safe and effective in the treatment of lower limb arteries2,3

For Challenging Patient Groups4
Excellent results despite a complex population at baseline4

Effective Drug Delivery
Reduction of drug loss with SafeGuard™ Insertion Aid1

Clinically Proven
Randomized controlled trials and all-comers registries have investigated safety and efficacy in the treatment of over 1,900 patients with peripheral artery disease (PAD) in the femoropopliteal and infrapopliteal arteries.
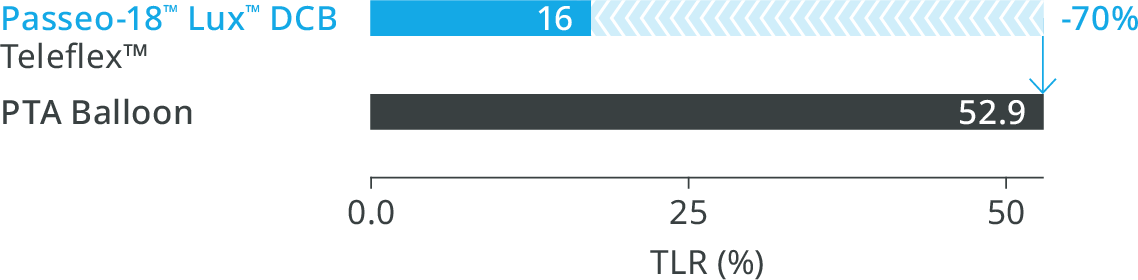
BIOLUX P-I RCT2 Femoropopliteal Indication10
Significantly reduced Target Lesion Revascularization (TLR) at 12 months compared to the control PTA* balloon in the as-treated population2
BIOLUX P-II RCT3 Infrapopliteal Indication
Major Adverse Events (MAE) rate of the Passeo™-18 Lux™ DCB was lower compared to the control PTA balloon.
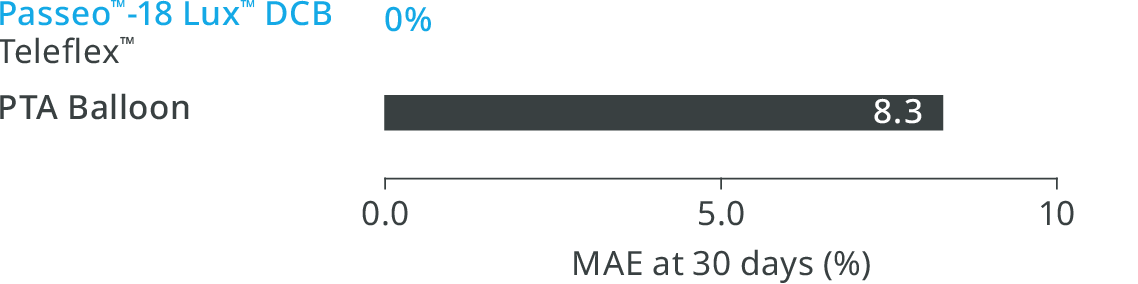
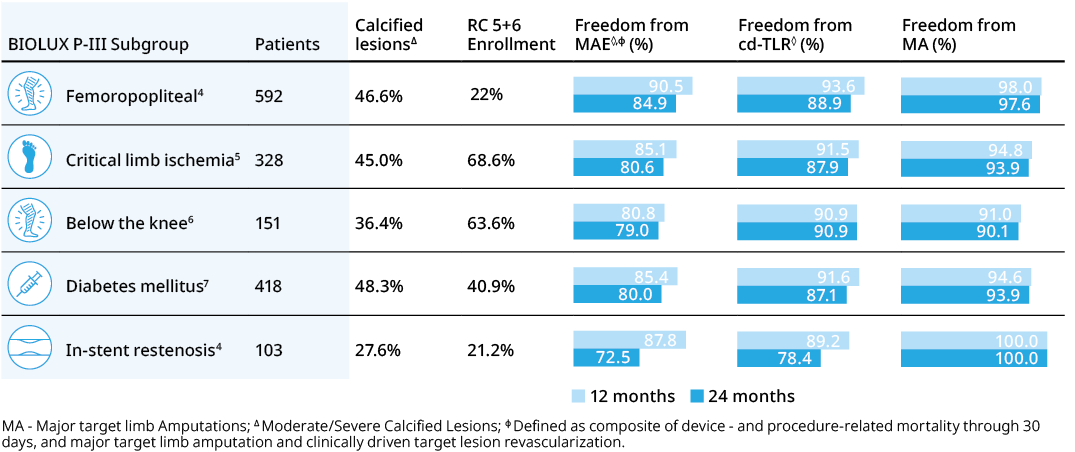
BIOLUX P-III4 All-Comers Registry
Passeo™-18 Lux™ DCB demonstrates excellent outcomes in one of the largest real-world DCB registries with few exclusion criteria.

For Challenging Patient Groups
Safety and efficacy clinically proven across challenging subgroups in BIOLUX P-III all-comers registry.

Effective Drug Delivery
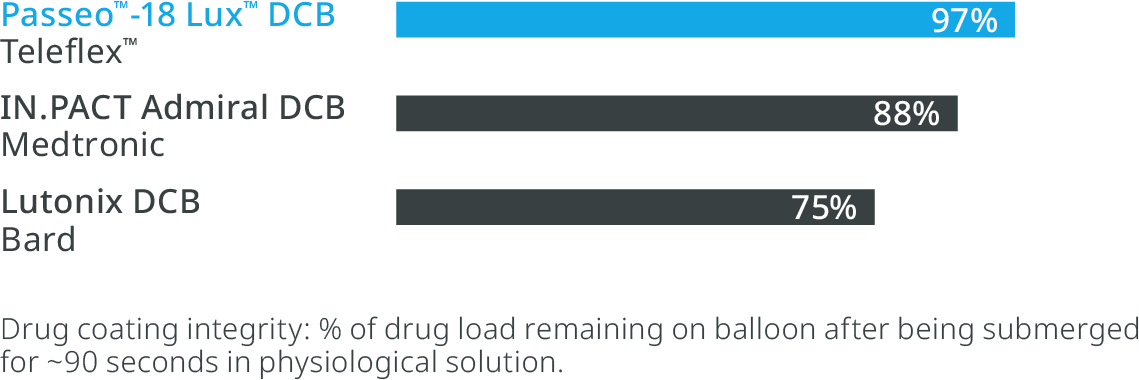
Effective drug delivery to the lesion with SafeGuard™ insertion aid and the hydrophobic BTHC excipient as part of the Lux™ Coating.
Reduction of drug loss in the introducer sheath valve4
The SafeGuard™ insertion aid improves ease of handling and protects the user and the Lux™ Coating on the balloon from contact and damage.

High drug retention1
Lux™ coating provides a hydrophobic BTHC excipient, which is less soluble than hydrophilic alternatives, ensuring more drug is available at the lesion site.
Clinical Highlights
BIOLUX P-I
84.6% Freedom from TLR at 12 months
Clinical trial to asses the safety and performance of the coated Passeo-18 Lux paclitaxel-releasing PTA balloon catheter versus the uncoated Passeo-18 balloon catheter for treatment of stenosis of the femoropopliteal arteries
See MoreBIOLUX P-II
0.0% MAE composite at 30 days
Clinical trial to asses the safety and performance of the coated Passeo-18 Lux paclitaxel-releasing PTA balloon catheter versus the uncoated Passeo-18 balloon catheter for treatment of stenosis of the femoropopliteal arteries
See MoreBIOLUX P-II
82.9% PP vs 73.9% compared to the control PTA balloon at 6 months
First-in-human study to assess the safety and performance of the Passeo-18 Lux drug coated balloon vs. the uncoated Passeo-18 catheter in patients with stenosis and occlusion of the infrapopliteal arteries
See MoreBIOLUX P-III
42.1% Critical limb ischemia patients
Prospective, international, multicenter, all-comers registry investigating safety and efficacy data on the Passeo-18 Lux DCB in a real-world population with atherosclerotic disease of the infaringuinal arteries
See MoreBIOLUX P-III
96.5% Freedom from Major Target Limb Amputation at 24 months
Prospective, international, multicenter, all-comers registry investigating safety and efficacy data on the Passeo-18 Lux DCB in a real-world population with atherosclerotic disease of the infaringuinal arteries
See MoreBIOLUX P-III
88.1% Freedom from cd-TLR at 24 months
Prospective, international, multicenter, all-comers registry investigating safety and efficacy data on the Passeo-18 Lux DCB in a real-world population with atherosclerotic disease of the infaringuinal arteries
See MoreProduct Overview
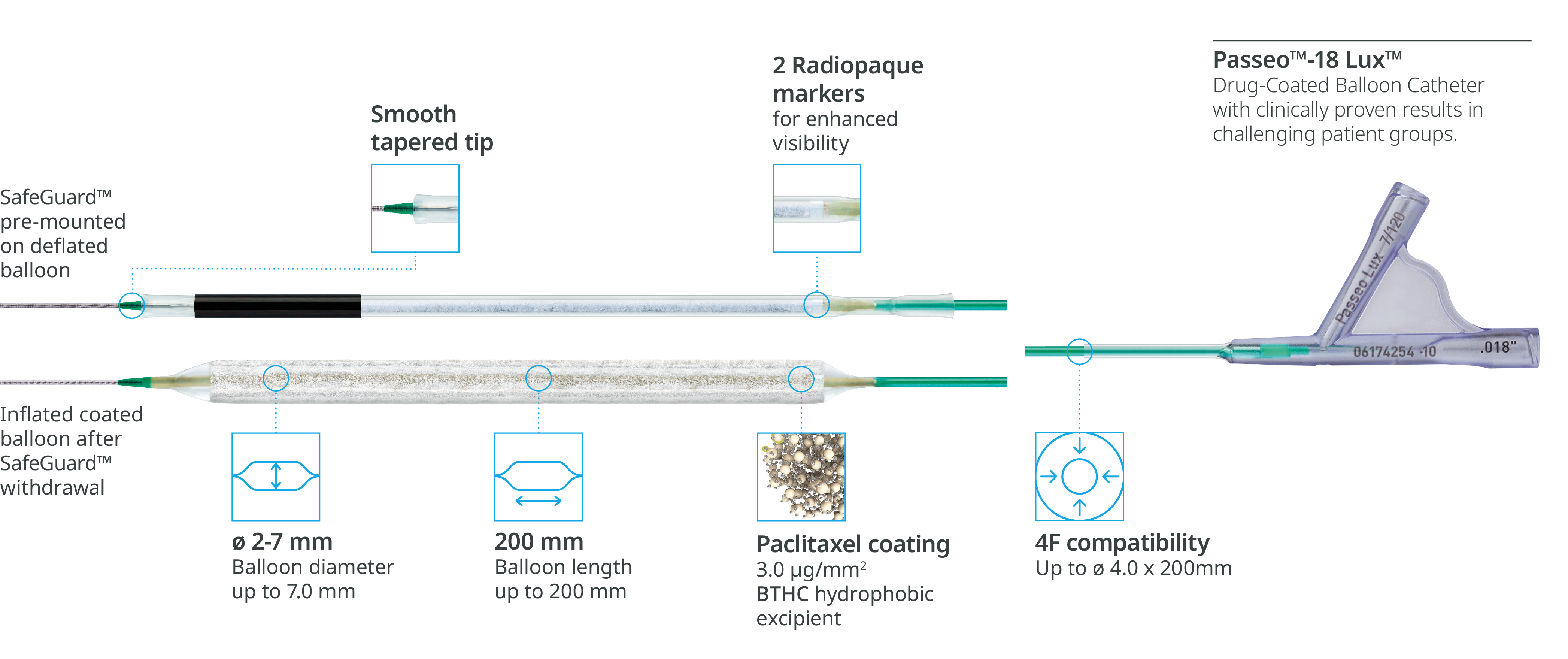
| Drug-coated balloon | |
| Catheter type | OTW |
|---|---|
| Recommended guide wire | 0.018” |
| Tip | Short, tapered |
| Balloon markers | 2 swaged markers (zero profile) |
| Shaft | 3.8F, hydrophobic coated |
| Usable Length | 90, 130 cm; 150 cm (only ø 2.0 mm) |
| Introducer size | 4F (ø 2.0 - 4.0 mm); 5F (ø 5.0 - 7.0 mm) |
| Nominal Pressure (NP) | 6 atm |
| Rated Burst Pressure (RBP) |
15 atm (ø 2.0-5.0 mm, L 40-120; ø 2.0-4.0 mm, L 150 mm); 14 atm (ø 2.0-3.0 mm, L 200 mm); 13 atm (ø 4.0-5.0 mm, L 200 mm); 12 atm (ø 5.0 mm, L 150 mm; ø 6.0, 7.0 mm) |
| Coating | |
| Drug | Paclitaxel |
|---|---|
| Drug concentration | 3.0 μg/mm2 |
| Coating matrix | Lux™ Coating comprising Paclitaxel and Butyryl-tri-hexyl citrate (BTHC) |
| Coated area | Cylindrical section of the balloon, exceeding the proximal and distal markers |
| Balloon Diameter * Length (mm) | |||||||||||||||||||||
| ø 2.0 x | ø 2.0 x | ø 2.5 x | ø 2.5 x | ø 3.0 x | ø 3.0 x | ø 4.0 x | ø 4.0 x | ø 5.0 x | ø 5.0 x | ø 5.0 x | ø 6.0 x | ø 7.0 x | |||||||||
| 40-150 | 200 | 40-150 | 200 | 40-150 | 200 | 40-150 | 200 | 40-120 | 150 | 200 | 40-200 | 40-200 | |||||||||
| Nominal Pressure (NP) | atm** | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | 6 | |||||||
| ø (mm) | 2 | 2 | 2.5 | 2.5 | 3 | 3 | 4 | 4 | 5 | 5 | 5 | 6 | 7 | ||||||||
| Rated Burst Pressure (RBP) | atm** | 15 | 14 | 15 | 14 | 15 | 14 | 15 | 13 | 15 | 12 | 13 | 12 | 12 | |||||||
| atm** ø (mm) | 2.1 | 2.1 | 2.6 | 2.6 | 3.2 | 3.2 | 4.3 | 4.2 | 5.3 | 5.2 | 5.2 | 6.2 | 7.3 | ||||||||
| **1 atm = 1.013 bar | |||||||||||||||||||||
| Catheter Length (cm) | Balloon ø (mm) | Balloon Length (mm) | ||||||||
| 40 | 60 | 80 | 100 | 120 | 150 | 200 | ||||
| 4F | 90 | 2.0 | 379860 | - | 379861 | - | 379862 | 449970 | 449977 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 90 | 2.5 | 379866 | - | 379867 | - | 379868 | 449971 | 449978 | ||
| 90 | 3.0 | 370843 | 484199 | 370848 | 484206 | 370853 | 449972 | 449979 | ||
| 90 | 4.0 | 370844 | 484200 | 370849 | 484207 | 370854 | 449973 | 449980 | ||
| 5F | 90 | 5.0 | 370845 | 484201 | 370850 | 484208 | 370855 | 449974 | 449981 | |
| 90 | 6.0 | 370846 | 484202 | 370851 | 484209 | 370856 | 449975 | 449982 | ||
| 90 | 7.0 | 370847 | 484203 | 370852 | 484210 | 370857 | 449976 | 449983 | ||
| 4F | 150 | 2.0 | 379863 | 484211 | 379864 | 484218 | 379865 | 449984 | 449991 | |
| 130 | 2.5 | 379869 | 484212 | 379870 | 484219 | 379871 | 449985 | 449992 | ||
| 130 | 3.0 | 370858 | 484213 | 370863 | 484220 | 370868 | 449986 | 449993 | ||
| 130 | 4.0 | 370859 | 484214 | 370864 | 484221 | 370869 | 449987 | 449994 | ||
| 5F | 130 | 5.0 | 370860 | 484215 | 370865 | 484222 | 370870 | 449988 | 449995 | |
| 130 | 6.0 | 370861 | 484216 | 370866 | 484223 | 370871 | 449989 | 449996 | ||
| 130 | 7.0 | 370862 | 484217 | 370867 | 484224 | 370872 | 449990 | 449997 | ||
IFU Link
References:
PTA - Percutaneous Transluminal Angioplasty; RCT: Randomized Controlled Trial; MAE: Major Adverse Events; cd-TLR: clinically driven Target Lesion Revascularization.
MA - Major target limb Amputations; Δ Moderate/Severe Calcified Lesions; ф Defined as composite of device - and procedure-related mortality through 30 days, and major target limb amputation and clinically driven target lesion revascularization.
BTHC = hydrophobic butyryl-tri-hexyl citrate
TLR = Target Lesion Revascularization; MAE = Major Adverse Event; PP = Primary Patency; PTA = Percutaneous Transluminal Angioplasty; BMS = Bare-Metal Stent; cd-TLR = clinically-driven Target Lesion Revascularization.
* Indication as per IFU. **Passeo-18 Balloon platform. Passeo-18 Lux Introducer size: 4F (⌀2.0-4.0 mm) ; 5F (⌀ 5.0-7.0 mm). BTHC = hydrophobic butyryl-tri-hexyl citrate
1. Data on file; 2. Scheinert D. Paclitaxel Releasing Balloon in femoropopliteal lesions using BTHC excipient: 12-month results from the BIOLUX P-I randomized trial. JEVT, 2015; 22(1): 14-21; 3. Zeller et al. Paclitaxel-Coated Balloon in Infrapopliteal arteries 12-month results from the BIOLUX P-II randomized trial. J Am Coll Cardiol Intv. 2015; 8:1614-22; 4. Tepe G. Paclitaxel-Coated Balloon Angioplasty for the Treatment of Infrainguinal Arteries: 24-Month Outcomes in the Full Cohort of BIOLUX P-III Global Registry. Cardiovasc Intervent Radiol.2021;44:207-217; 5. Brodmann B et al. Real-World Experience With a Paclitaxel-Coated Balloon in Critical Limb Ischemia 24-Month Subgroup Outcomes of BIOLUX P-III. JACC Cardiovasc Interv. 2020;13:2289-2299; 6. Tepe G et al. BIOLUX P-III Passeo-18 Lux All-Comers Registry: 24-Month Results in Below-the-Knee Arteries. Cardiovasc Intervent Radiol. 2021;44:10-18; 7. Mwipatayi P, Barry I, Brodmann M, et al. Twenty-Four-Month Outcomes of Drug-Coated Balloon in Diabetic Patients in the BIOLUX P-III Registry: A Subgroup Analysis. Annals of Vascular Surgery (2021); https://doi.org/10.1016/j.avsg.2021.02.050.
The Passeo-18 Lux DCB with its Lux coating is part of the Lux family of Paclitaxel-coated balloons.