With usable lengths up to 170cm, Dynetic™-35 stent system allows a radial approach for the treatment of de novo or restenotic atherosclerotic lesions in iliac arteries.*
Product Highlights
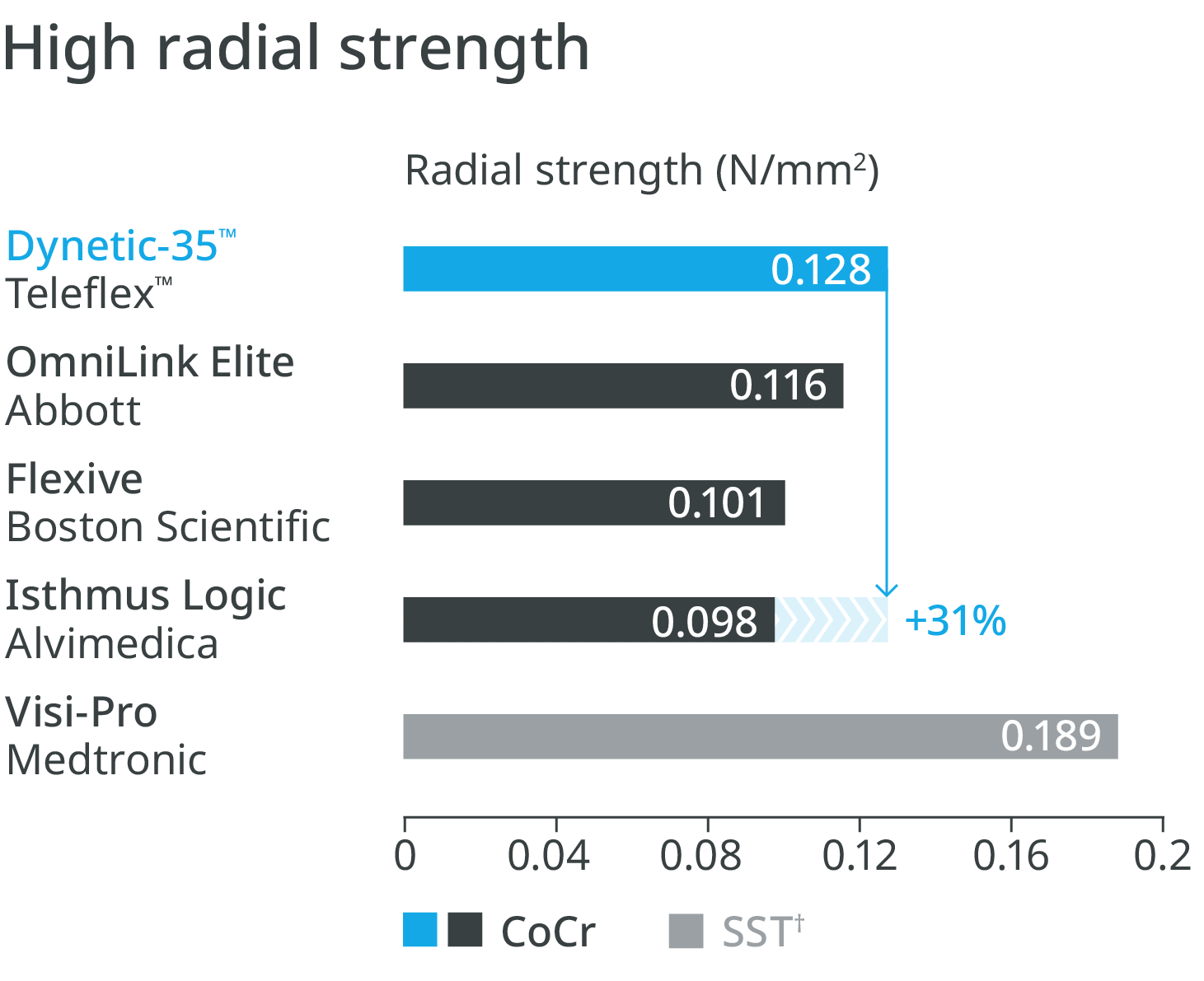
High radial strength1
Thin struts, super flexible2 Cobalt Chromium stent design
Largest size range3
Complete size range with diameters of 5.0 – 10.0 mm and lengths of 18 – 78 mm
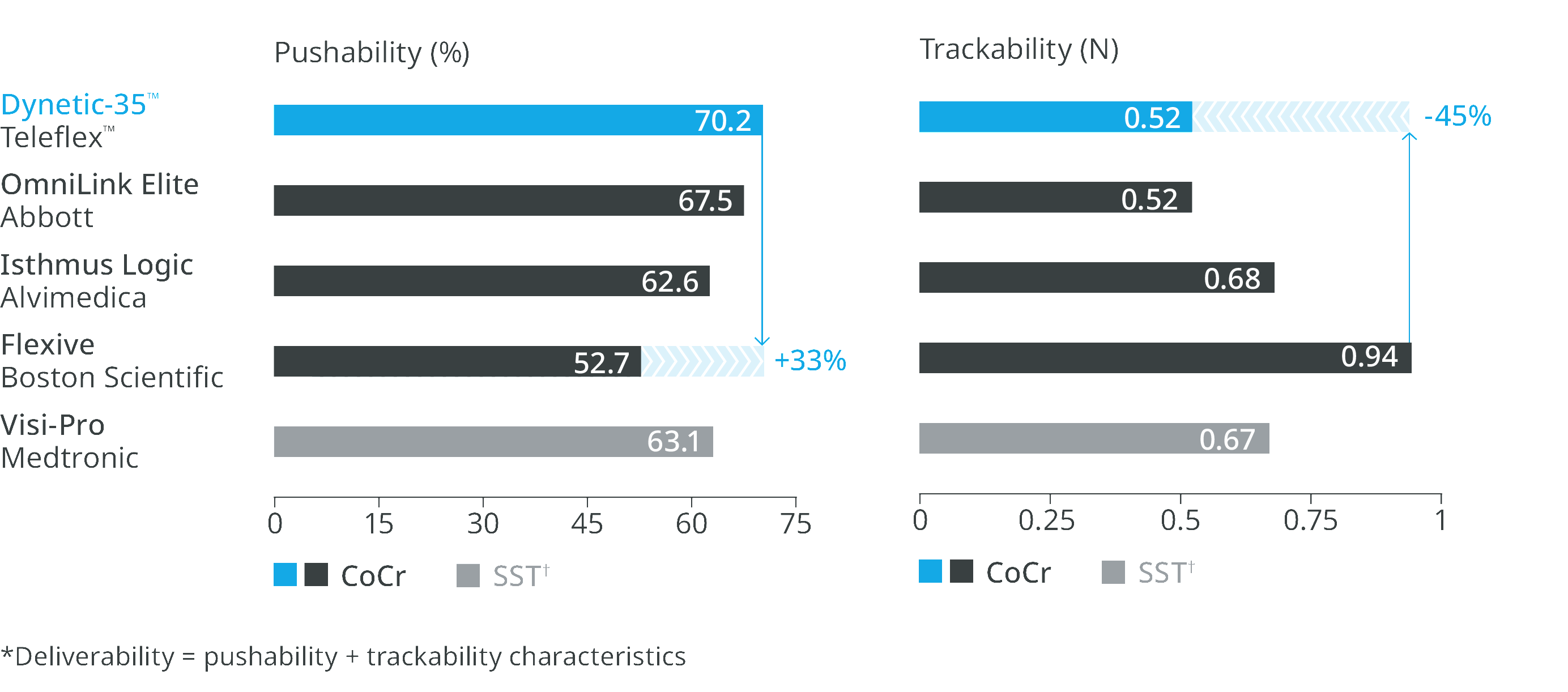
Deliverability in 6F compatibility4
Low profile balloon catheter with excellent deliverability1
High radial strength1
Thin struts with high radial strength
Higher radial strength compared to leading competitor Cobalt Chromium (CoCr) stents.1
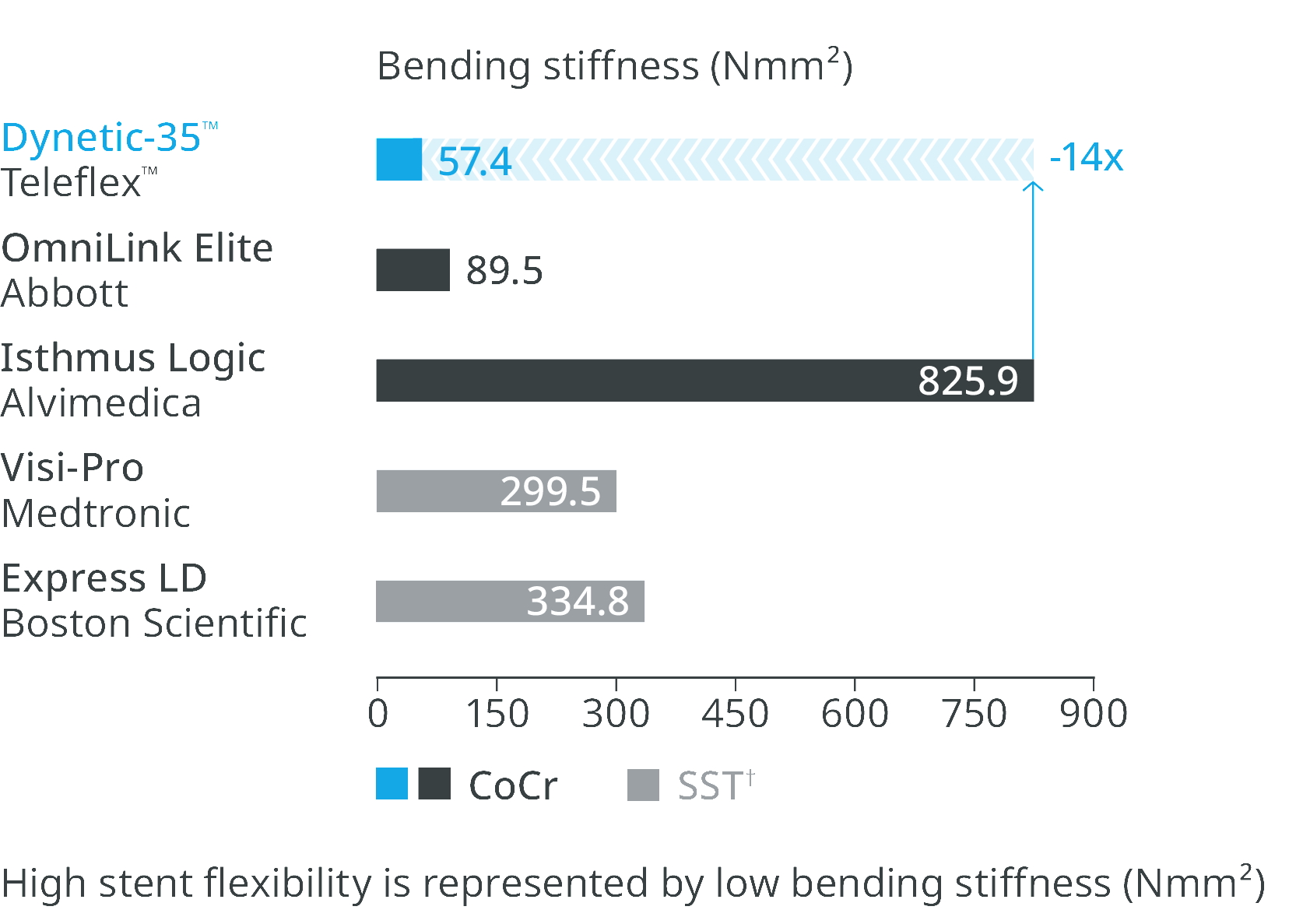
Super flexible stent design
Most flexible stent design compared to leading competitor stents.5
Largest size range
6F compatible in full size range
The thin strut stent design combined with the low profile balloon catheter delivery system offers the full product range in 6F sheath compatibility.4

Deliverability in 6F compatibility4
Excellent deliverability
The low profile design with its low crossing profile offers excellent deliverability* compared to leading competitor devices.1,4
Product Overview

| Technical Data | |
|---|---|
| Stent | Balloon-Expandable |
| Stent material | Cobalt Chromium |
| Strut thickness | 110 µm (ø 5.0 - 7.0 mm), 140 µm (ø 8.0 - 10.0 mm) |
| Shortening | Negligible |
| Stent coating | proBIO™ (Amorphous Silicon Carbide) |
| Sizes | ø 5.0 - 10.0 mm; L: 18 - 28 - 38 - 58 - 78 mm |
| Delivery system | |
|---|---|
| Catheter type | OTW |
| Recommended guide wire | 0.035” |
| Tip | Low entry profile, colored |
| Balloon markers | 2 swaged markers |
| Shaft | 5.1 - 5.4F, hydrophobic coating, dual-lumen |
| Usable length | 90 cm, 130 cm and 170 cm |
| Markers | 2 swaged markers |
| Guide wire lumen | Hydrophobic coating |
| Nominal Pressure (NP) | 10 atm |
| Rated Burst Pressure (RBP) | 14 atm (ø 5.0 - 8.0 mm), 12 atm (ø 9.0 - 10.0 mm) |
| Ordering Information — Catheter length 90 cm | |||||
|---|---|---|---|---|---|
| Stent ø (mm) | 18 | 28 | 38 | 58 | 78 |
| 5 | 428690 | 428694 | 428700 | 428706 | - |
| 6 | 428691 | 428695 | 428701 | 428707 | - |
| 7 | 428692 | 428696 | 428702 | 428708 | 428712 |
| 8 | 448939 | 448942 | 448945 | 448948 | 448951 |
| 9 | - | 448943 | 448946 | 448949 | 448952 |
| 10 | - | 448944 | 448947 | 448950 | 448953 |
| Ordering Information — Catheter length 130 cm | |||||
|---|---|---|---|---|---|
| Stent ø (mm) | 18 | 28 | 38 | 58 | 78 |
| 5 | 428716 | 428720 | 428726 | 428732 | - |
| 6 | 428717 | 428721 | 428727 | 428733 | - |
| 7 | 428718 | 428722 | 428728 | 428734 | 428738 |
| 8 | 448954 | 448957 | 448960 | 448963 | 448966 |
| 9 | - | 448958 | 448961 | 448964 | 448967 |
| 10 | - | 448959 | 448962 | 448965 | 448968 |
| Ordering Information — Catheter length 170 cm | |||||
|---|---|---|---|---|---|
| Stent ø (mm) | 18 | 28 | 38 | 58 | 78 |
| 5 | - | - | 428752 | - | - |
| 6 | - | 428747 | 428753 | 428759 | - |
| 7 | - | 428748 | 428754 | 428760 | 428764 |
| 8 | - | 448972 | 448975 | 448978 | 448981 |
| 9 | - | 448973 | 448976 | 448979 | - |
| 10 | - | - | 448977 | - | - |