PK Papyrus™ covered coronary stent is indicated for acute coronary artery perforations.a
Delivers like a conventional stent, allowing to seal coronary artery perforations with confidence.1

Product Highlights

Clinically provenb,3-15
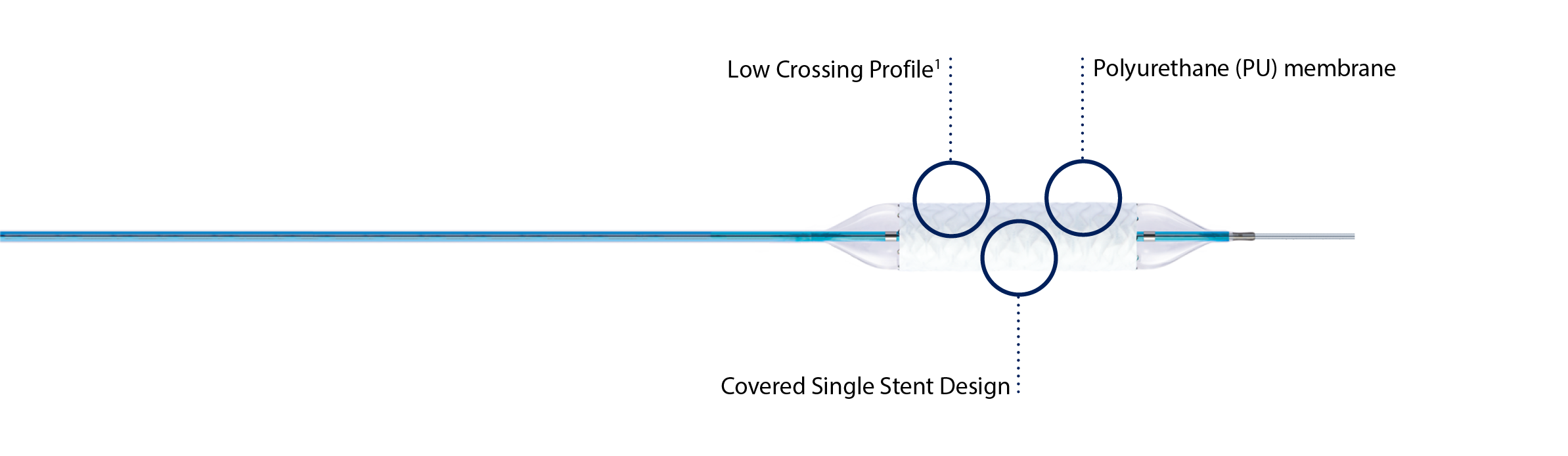
Covered single stent design1
Exceptional deliverabilityc,1,3

Clinically provenb,3-15
>1,500
Patients with PK Papyrus™ studied in clinical studies.16
10
Number of publications available.3-12
93.7%
Patients were not referred to emergency cardiac surgery.d,7
PK Papyrus™ is safe and effective as confirmed by one of the largest patient datasets for covered stents.e,7
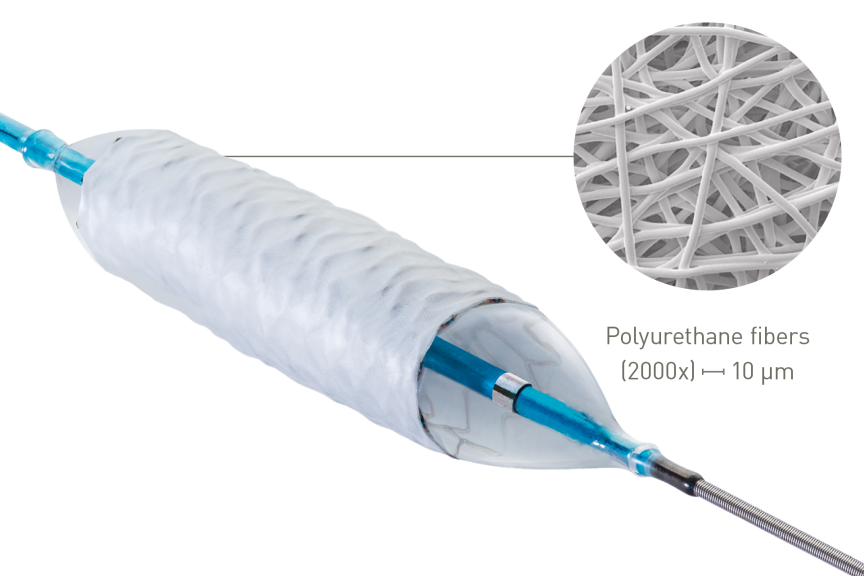
Covered single stent design
Innovative polyurethane membrane1
Unique manufacturing method allows for an ultra thin membrane capable of sealing vessel perforations1 and achieve a true covered single stent design1. Expect a high performing platform with an ultra thin membrane to deliver like a conventional stent.1
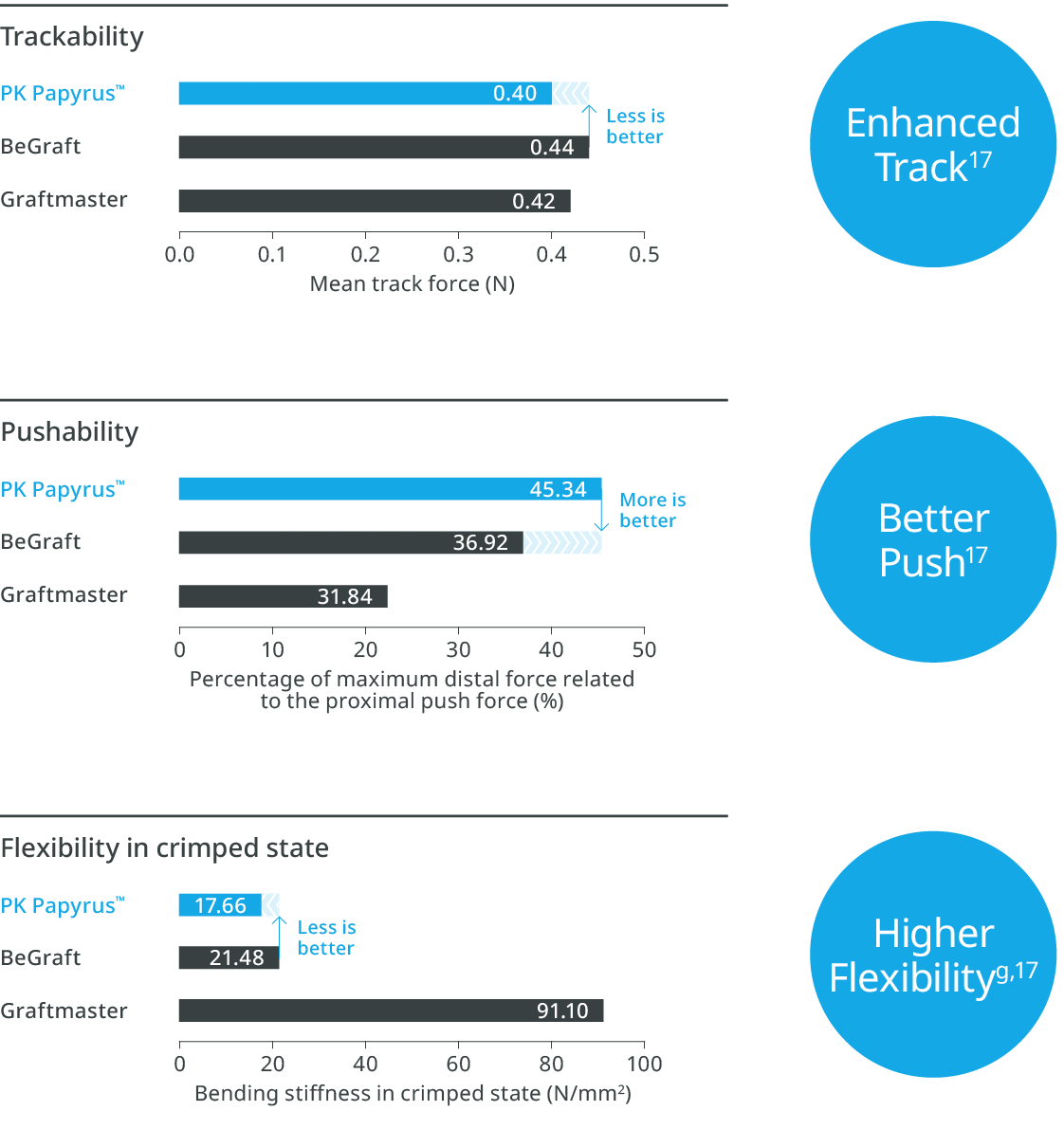
Exceptional deliverabilityc,1,3
Consistently high stent delivery successf,3-7
PK Papyrus™ is associated with reliable delivery (97.7% stent delivery success) and successful sealing (92.1%), favored by its unique design.7
Clinical Highlights
HDE POST-MARKET SURVEILLANCE SURVEY7
94.2% Successful following delivery
Clinical experience of the PK Papyrus covered stent in patients with coronary artery perfortations: Results from a multi-center Humanitarian Device Exemption (HDE) survey
See MoreSCAAR4
The only covered stent with > 90% procedural success rate
Observational srudy comparing stents with the overall stented population.
See MorePAST-PERF REGISTRY5
Low TLR (7.7%) and low define ST (3.3%) at 12 months
International, investigator-initiated, retrospective multicenter registry to collect data on PK Papyrus covered stent
See MoreProduct Overview
| Stent | |
|---|---|
| Stent cover material | Non-woven, electrospun polyurethane |
| Stent strut thickness | ø 2.5 - 3.0 mm: 60 μm (0.0024”)
ø 3.5 - 4.0 mm: 80 μm (0.0031”) ø 4.5 - 5.0 mm: 120 μm (0.0047”) |
| Stent Material | Cobalt chromium (L-605) with proBIO™
(Amorphous Silicon Carbide) coating |
| Maximum stent expansion diameter | ø 2.5 - 3.0 mm: 3.50 mm
ø 3.5 - 4.0 mm: 4.65 mm ø 4.5 - 5.0 mm: 5.63 mm |
| Delivery System | |
|---|---|
| Guide wire diameter | 0.014” |
| Usable catheter length | 140 cm |
| Recommended guide catheter | ø 2.5 - 4.0 mm: 5F (min. I.D.* 0.056”)
ø 4.5 - 5.0 mm: 6F (min. I.D.* 0.070”) |
| Nominal pressure (NP) | ø 2.5 - 3.5 mm: 8 atm
ø 4.0 - 5.0 mm: 7 atm |
| Rated burst pressure (RBP) | ø 2.5 - 4.0 mm: 16 atm
ø 4.5 - 5.0 mm: 14 atm |
| *I.D. = Inner Diameter | |
| Stent | Catheter length 140 cm | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ø (mm) | (Stent length mm) | |||||||||||||||
| 15 | 20 | 26 | ||||||||||||||
| 5F | 2.5 | 369380 | 369386 | |||||||||||||
| 3 | 369381 | 369387 | 381789 | |||||||||||||
| 3.5 | 369382 | 369388 | 381790 | |||||||||||||
| 4 | 369383 | 369389 | 381791 | |||||||||||||
| 6F | 4.5 | 369384 | 369390 | 369392 | ||||||||||||
| 5 | 369385 | 369391 | 369393 | |||||||||||||