Orsiro™ Mission™ DES is indicated for improving coronary luminal diameter in patients with symptomatic ischemic heart disease due to discrete de-novo stenotic lesions and in-stent restenotic lesions (length ≤ 40 mm) in the native coronary arteries with a reference vessel diameter of 2.25 mm to 4.0 mm including the following patient and lesion subsets:
- Acute Coronary Syndrome (ACS)
- ST-Elevation Myocardial Infarction (STEMI)
- Diabetes Mellitus (DM)
- High Bleeding Risk (HBR)
- One Month of Dual Antiplatelet Therapy (DAPT) in HBR Patients
- Calcified Lesions (moderate/severe calcification)
- Complex Lesions (B2/C)
- Long Lesions (LL) (e.g. ≥ 20 mm)
- Small Vessels (SV) (e.g. ≤ 2.75 mm)
- Multi-Vessel Disease (MVD)
- Male/Female
- Old Patients (e.g. > 65 y)
Product Highlights
The next level of deliverability2
1st in Push3, 1st in Track3, 1st in Cross3
Ultrathin struts4
For early endothelialization5
Outstanding patient outcomes6,b
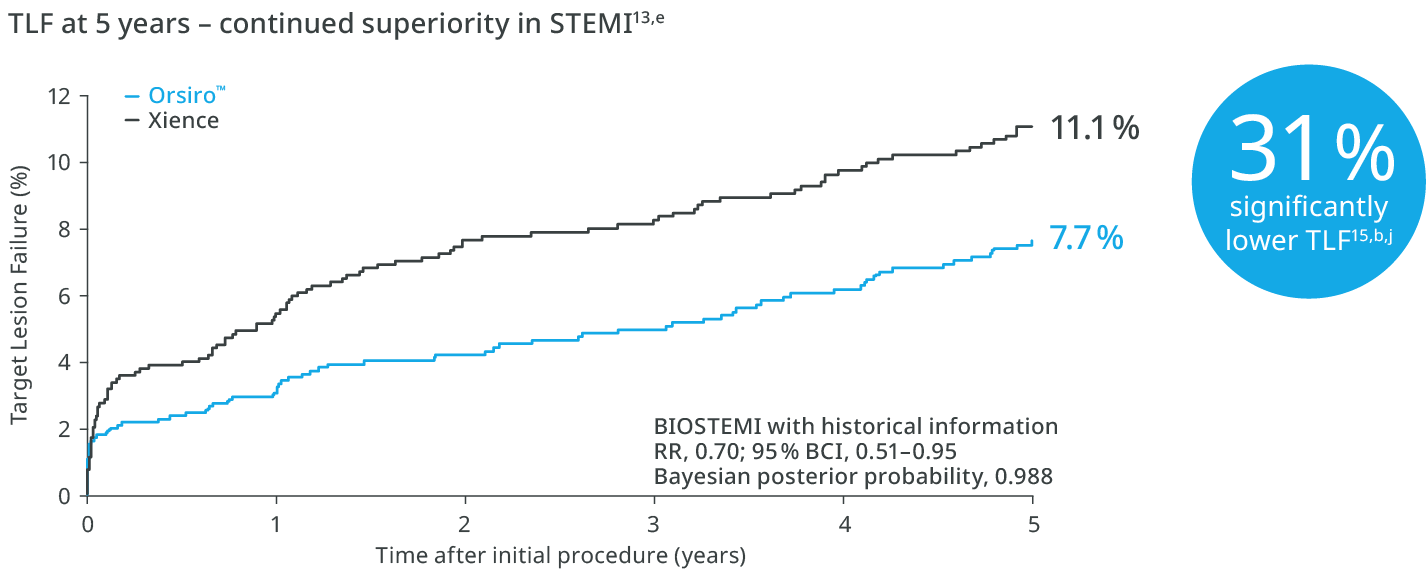
Proven superiority in STEMI up to 5 years1,b,c
The next level of deliverability2
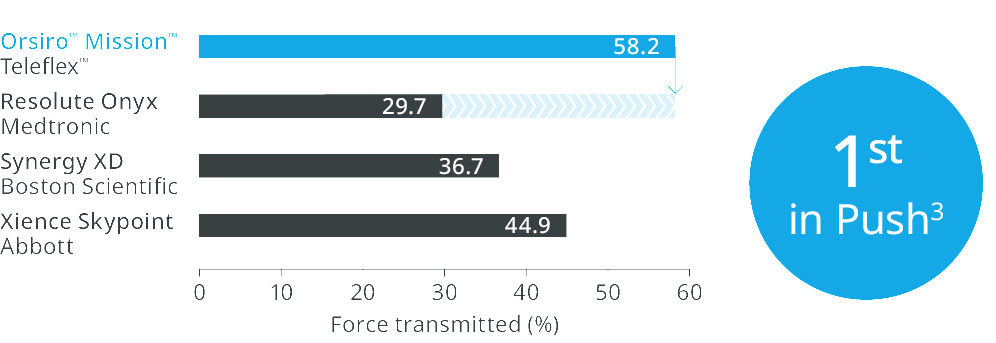
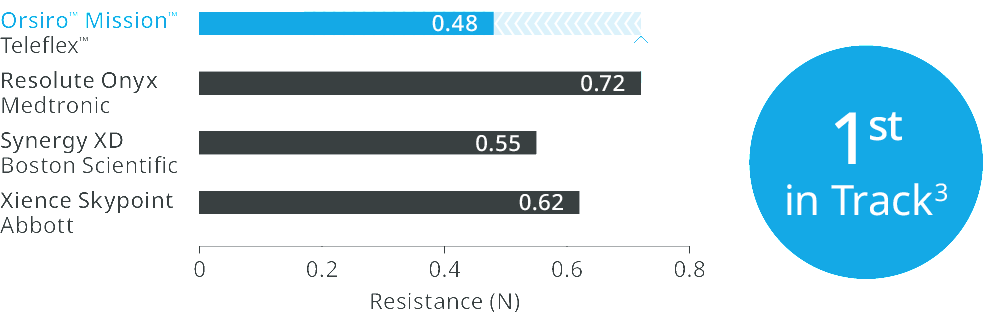
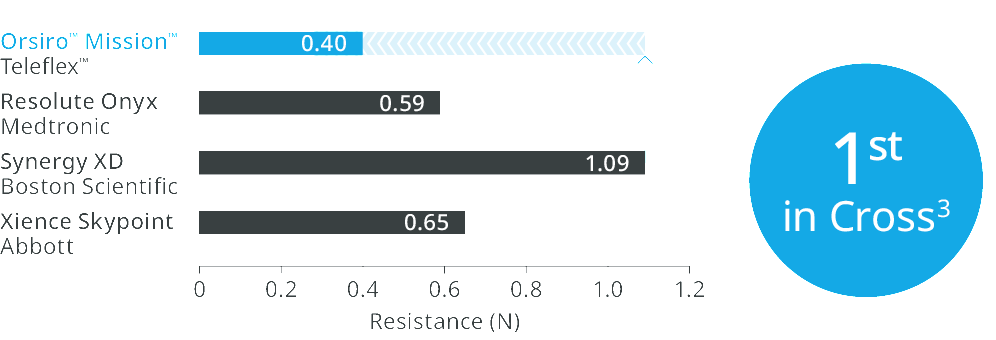
Orsiro™ Mission™ DES transmits up to 96% more force from hub to tipd, requires 33% less force to follow the path to the lesiond, and 64% less force to crosse.
1st in Push3
Transmitting up to 96% more force from hub to tip.
1st in Track3
Up to 33% less force needed to follow the path to the lesion.
1st in Cross3
Up to 64% less force needed to successfully cross demanding anatomies.
Ultrathin struts4
For early endothelialization.5
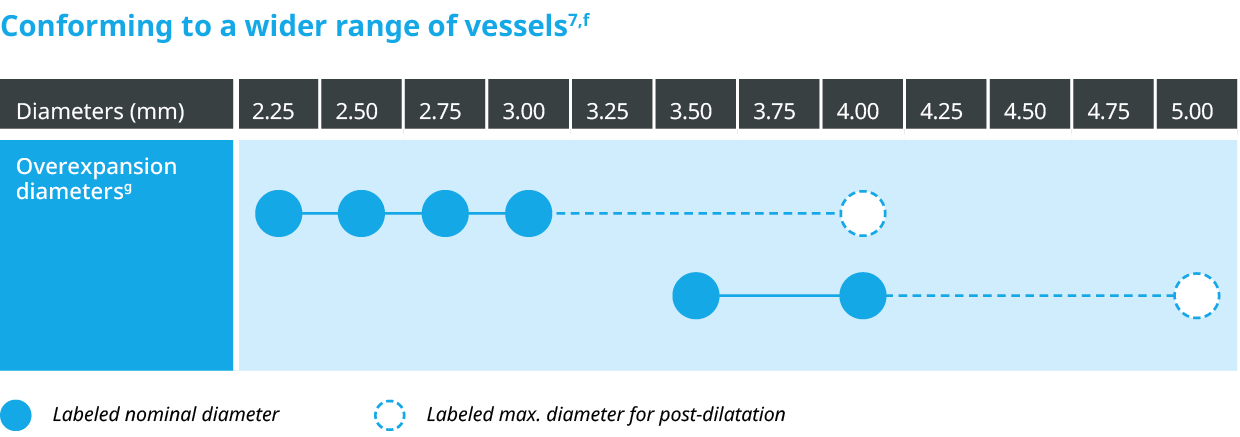
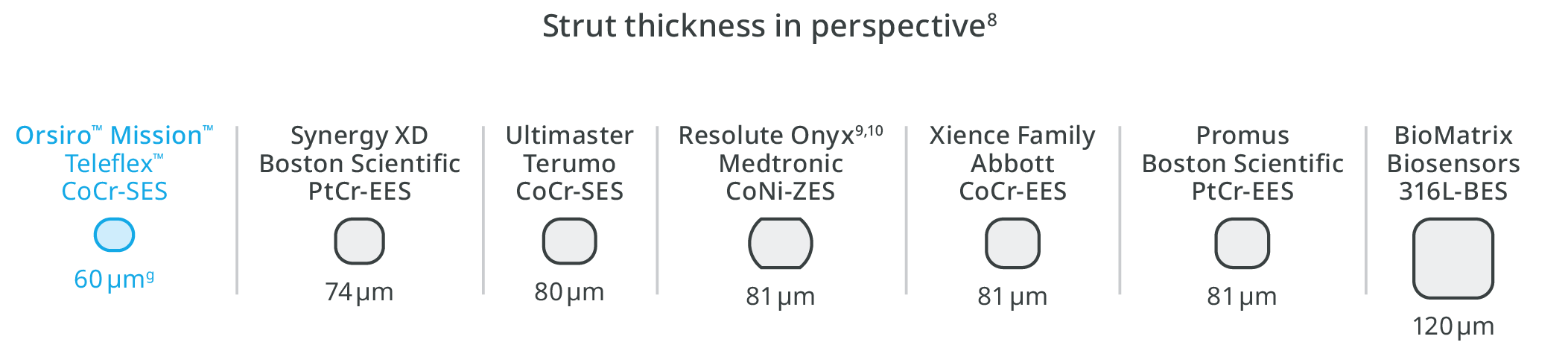
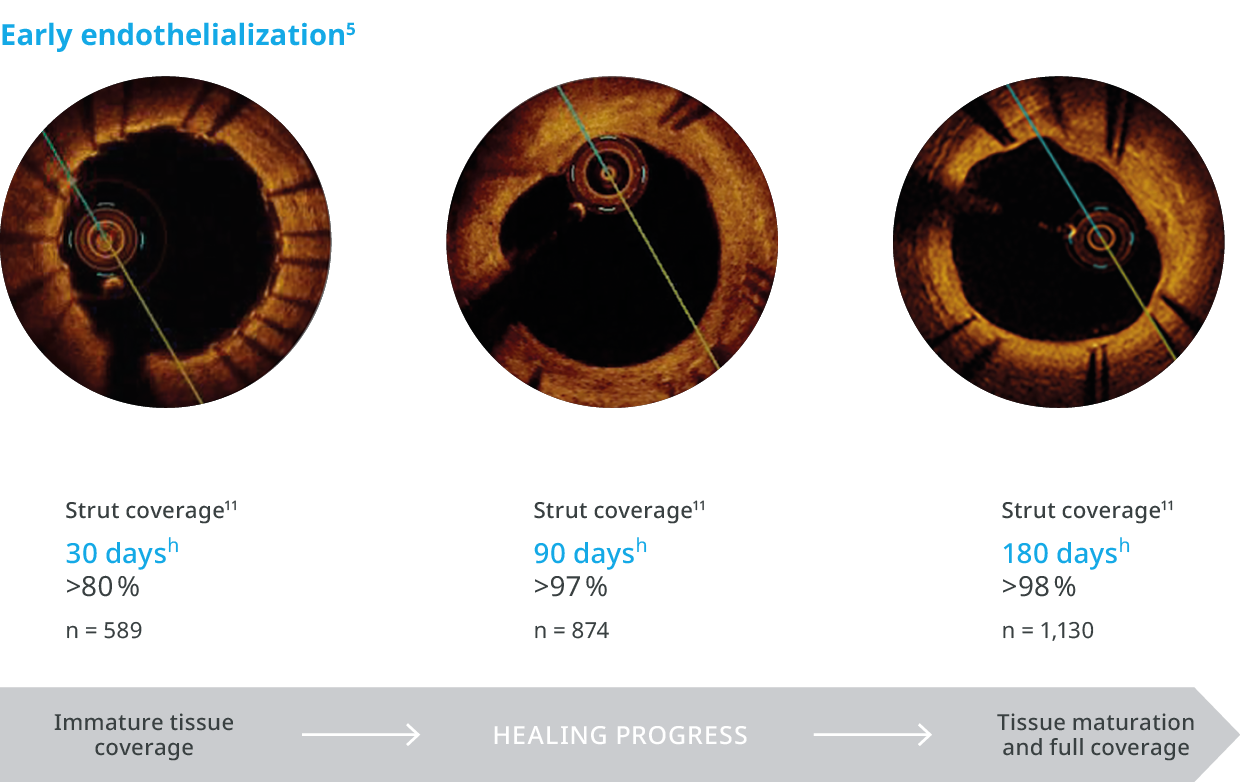
Outstanding patient outcomes6,b
Orsiro™ DES proves continued superiority in the first randomized controlled trial demonstrating superiority between two contemporary DES.12
Orsiro™ family of DES – One of the most studied DES 14,b,i

Clinical Highlights
BIOFLOW-V16
36% significantly lower TV-MI with Orsiro™
Comparison of Ultrathin Sirolimus-Eluting Bioresorbable Polymer with Thin Everolimus-Eluting Durable Polymer Stents.
See MoreBIOSTEMI1
31% lower risk of TLF with Orsiro™
Investigator-initiated, prospective, multicentre, assessor-blinded, randomized (1:1), controlled. Superiority trial comparing Orsiro and Xience in STEMI patients undergoing primary PCI.
See MoreBIOFLOW-DAPT17
Orsiro™ Mission™ DES showed safety and efficacy for short-DAPT
Prospective, multi-center, international, two-arm randomized controlled clinical study, including a total of 1,948 subjects.
See MoreTAGLIERI NETWORK MA18
70.8% probability for Orsiro™ to rank as best stent
Network meta-analysis of randomized controlled trials comparing Orsiro™ and 9 other currently used drug-eluting stents.
See MoreProduct Overview

| Stent | |
|---|---|
| Stent material | Cobalt chromium, L-605 |
| Strut thickness | ø 2.25 – 3.0 mm: 60 µm (0.0024”); ø 3.50 – 4.0 mm: 80 µm (0.0031”) |
| Passive coating | proBIO™ (Amorphous Silicon Carbide) |
| Active coating | BIOlute™ bioabsorbable Poly-L-Lactide (PLLA) eluting a limus drug |
| Drug dose | 1.4 µg/mm2 |
| Delivery System | |
| Catheter type | Rapid exchange |
| Recommended guide catheter | 5F (min. I.D. 0.056”) |
| Guide wire diameter | 0.014” |
| Usable catheter length | 140 cm |
| Balloon material | Semi crystalline polymer material |
| Coating (Distal shaft) | Hydrophilic |
| Coating (Proximal shaft) | Hydrophobic |
| Marker bands | Two swaged platinum-iridium markers |
| Lesion entry profile | 0.017” |
| Distal shaft diameter | 2.7F: ø 2.25 – 3.0 mm; 2.9F: ø 3.5 – 4.0 mm |
| Proximal shaft diameter | 2.0F |
| Nominal pressure (NP) | 10 atm |
| Rated burst pressure (RBP) | 16 atm |
| Storage | |
|---|---|
| Use Before Date (UBD) | 36 months |
| Temperature | Between 15°C (59°F) and 25°C (77°F), short term
excursions between 10°C (50°F) and 40°C (104°F) are allowed |
| Double Helix Stent Design | ||||||
|---|---|---|---|---|---|---|
| Nominal diameter (mm) | 2.25 | 2.5 | 2.75 | 3 | 3.5 | 4 |
| Strut thickness (µm) | 60 | = | = | = | 80 | = |
| Strut width (µm) | 75 | = | = | = | 85 | = |
| Amount of connectors | 3 | = | = | = | 3 | = |
| Amount of crowns at end | 8 | = | = | = | 8 | = |
| Maximal Expansion and Stent Strut Opening | ||||||
|---|---|---|---|---|---|---|
| Nominal diameter (mm) | 2.25 | 2.5 | 2.75 | 3 | 3.5 | 4 |
| Nominal outer diameter of the stent at NP (mm) | 2.37 | 2.62 | 2.87 | = | = | = |
| Maximal expansion diameter (mm) | 4 | = | = | = | 5 | = |
| Stent strut opening diameter at NP* (mm) | 0.79 | 0.92 | = | = | 1.06 | 1.25 |
| Maximal diameter of expanded stent cell (mm) | 3.59 | = | = | = | 4.42 | = |
*Mean of the largest possible opening diameter within a stent cell at NP
= symbol used to show repetition
| Stent ø (mm) | Stent Length (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 9 | 13 | 15 | 18 | 22 | 26 | 30 | 35 | 40 | ||
| 2.25 | 419101 | 419107 | 419113 | 419119 | 419125 | 419131 | 419137 | 419143 | 419149 | |
| 2.5 | 419102 | 419108 | 419114 | 419120 | 419126 | 419132 | 419138 | 419144 | 419150 | |
| 2.75 | 419103 | 419109 | 419115 | 419121 | 419127 | 419133 | 419139 | 419145 | 419151 | |
| 3 | 419104 | 419110 | 419116 | 419122 | 419128 | 419134 | 419140 | 419146 | 419152 | |
| 3.5 | 419105 | 419111 | 419117 | 419123 | 419129 | 419135 | 419141 | 419147 | 419153 | |
| 4 | 419106 | 419112 | 419118 | 419124 | 419130 | 419136 | 419142 | 419148 | 419154 | |