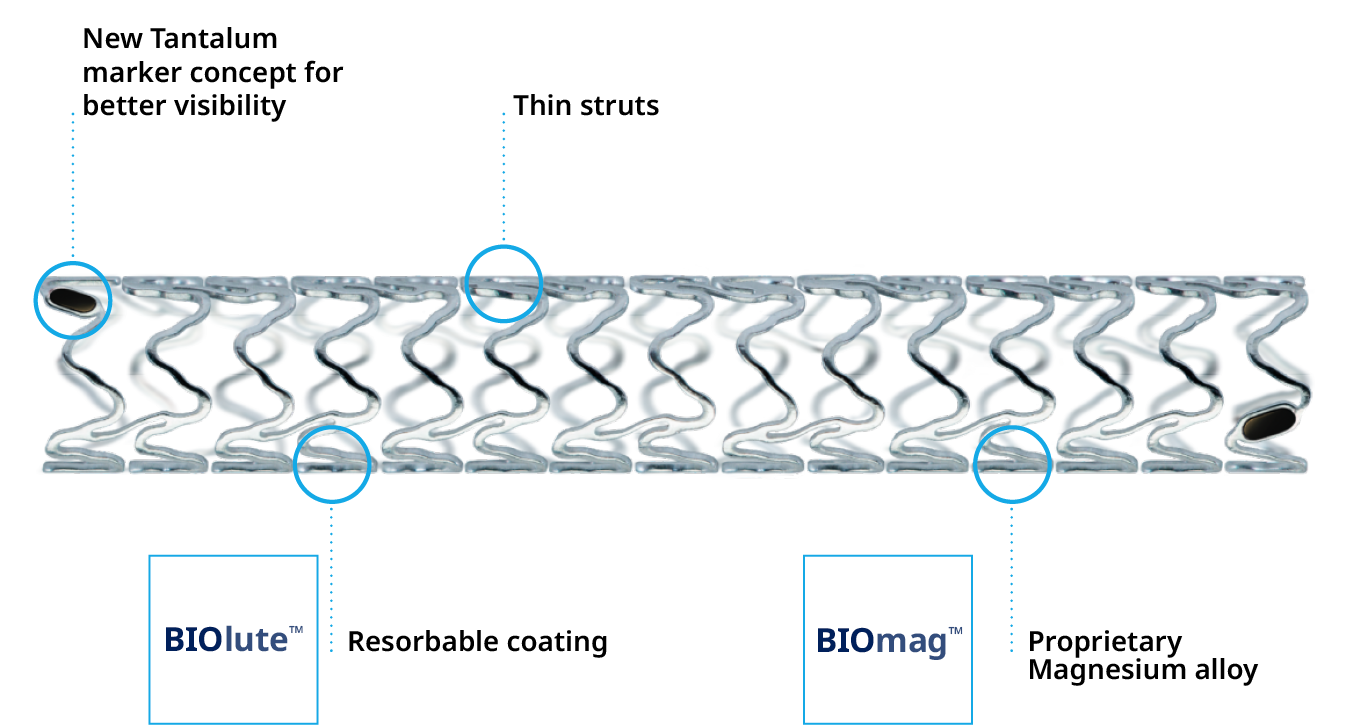
Product Highlights
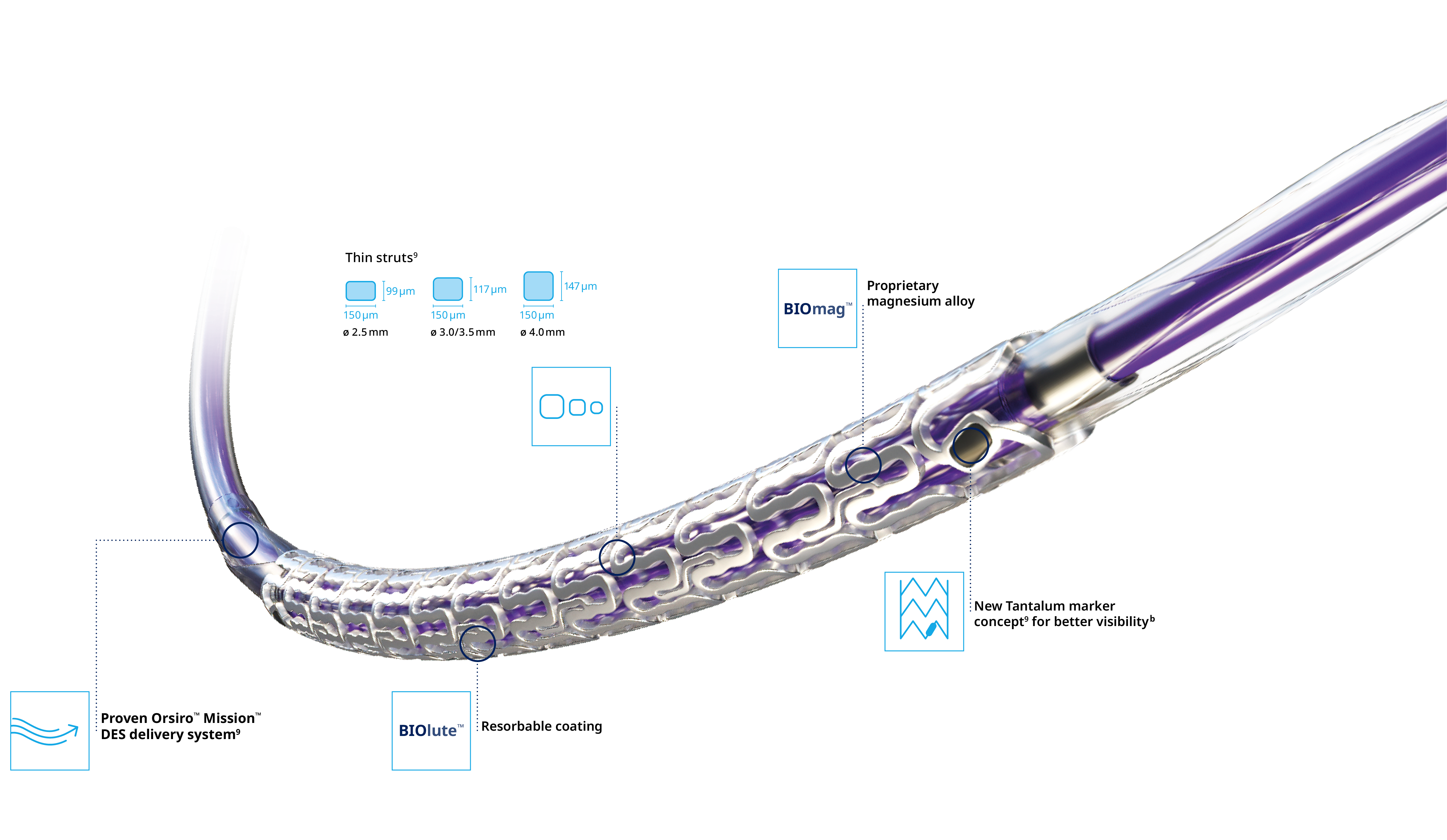
Delivers like a DES5
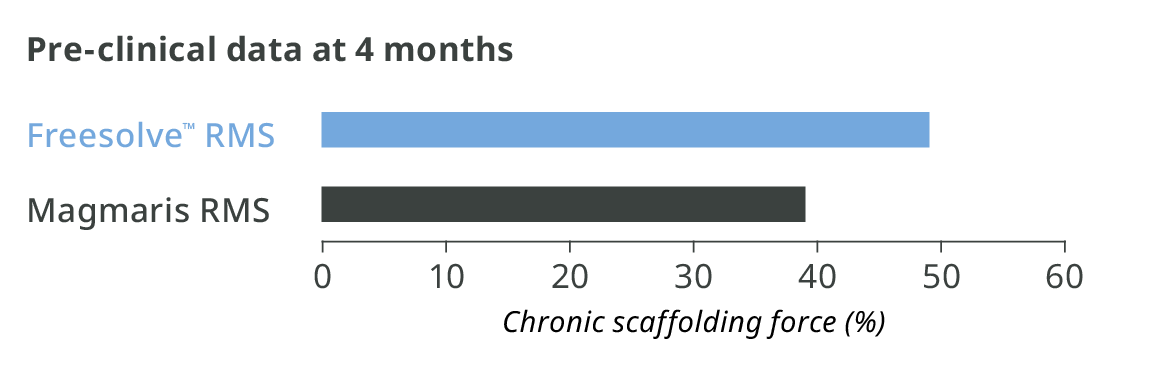
Optimal vessel support6,7
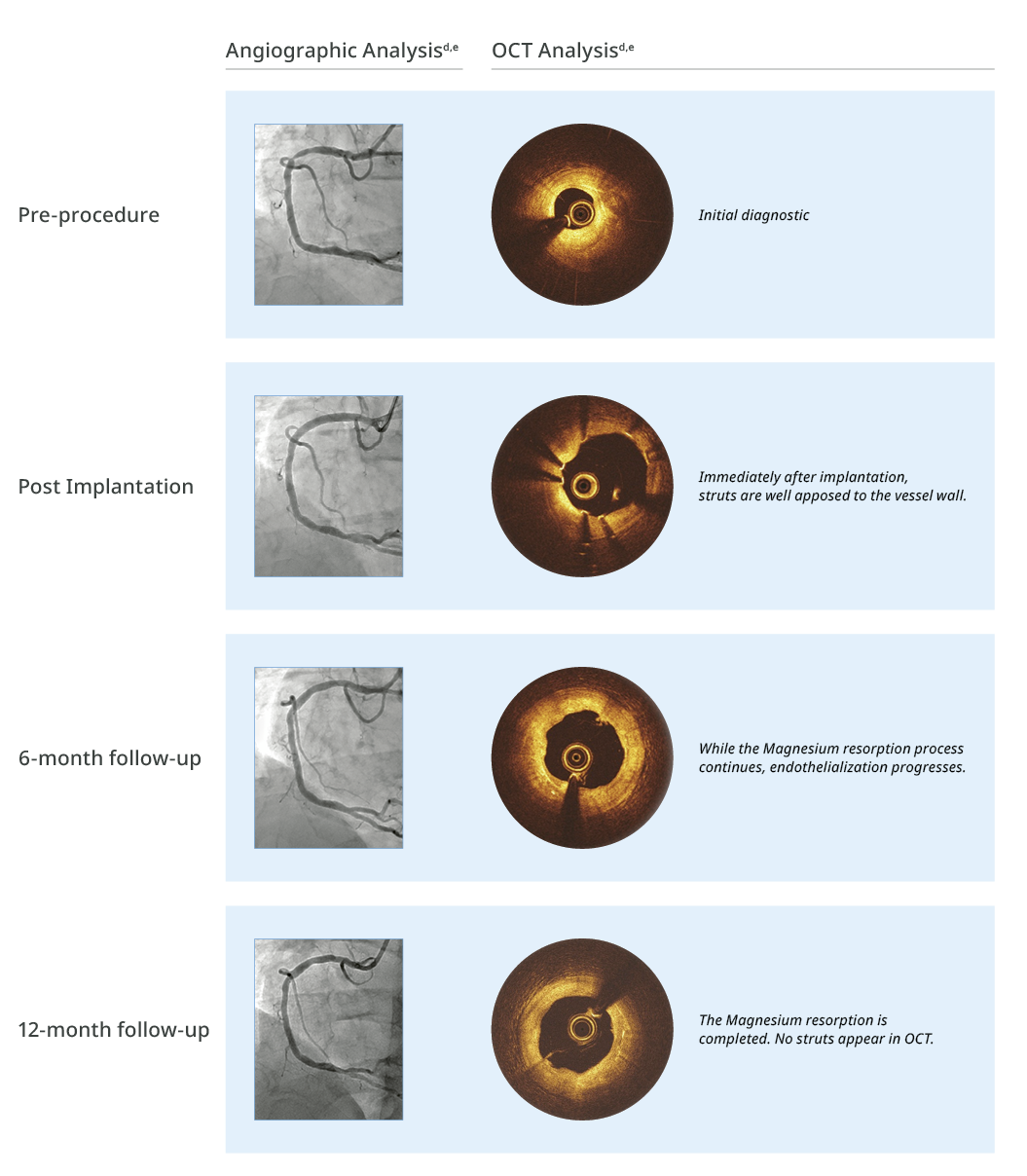
Magnesium fully resorbed after 12 months8
Excellent safety and efficacy2,3
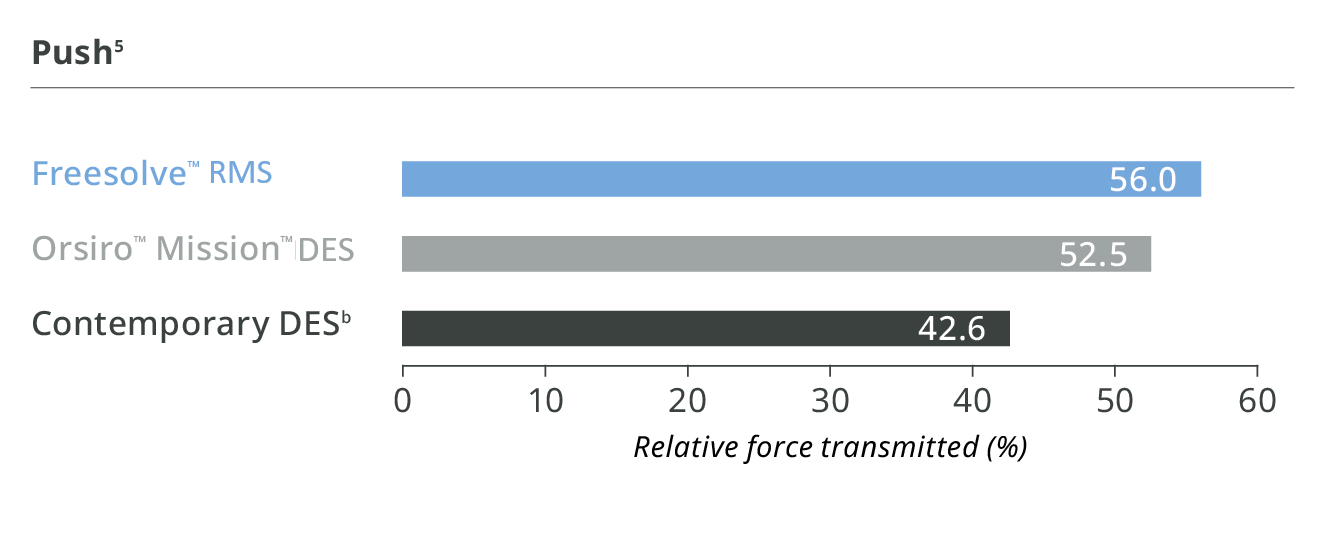
Better than contemporary DES
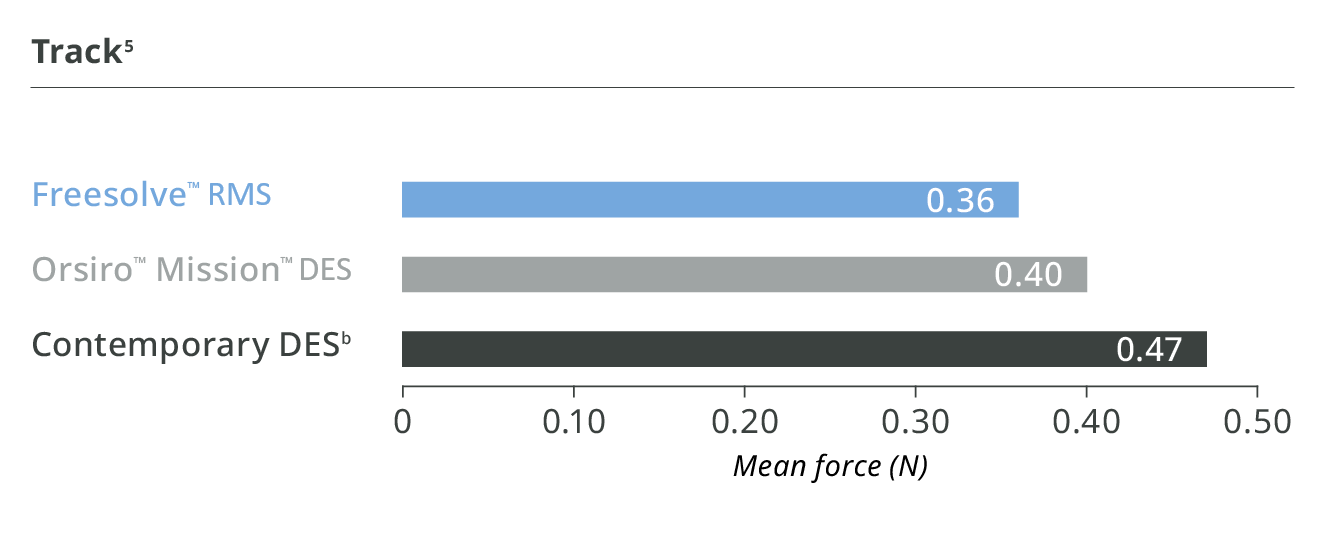
Better than contemporary DES
Predictable, homogeneous resorption process6
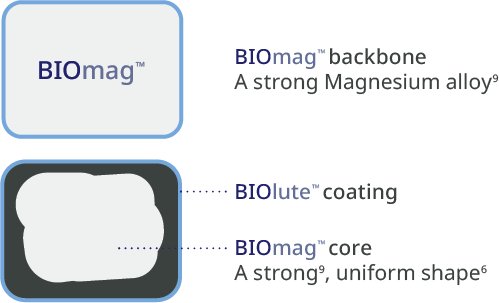
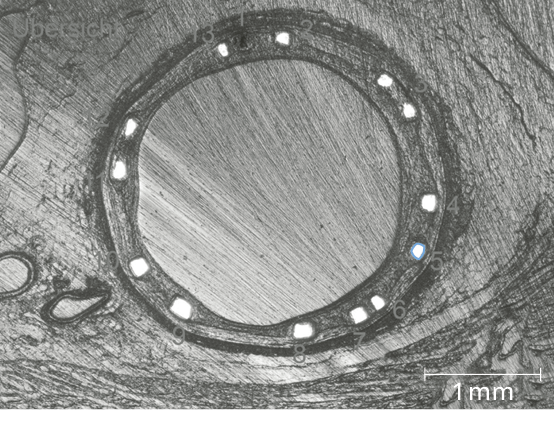
Equal resorption between struts6
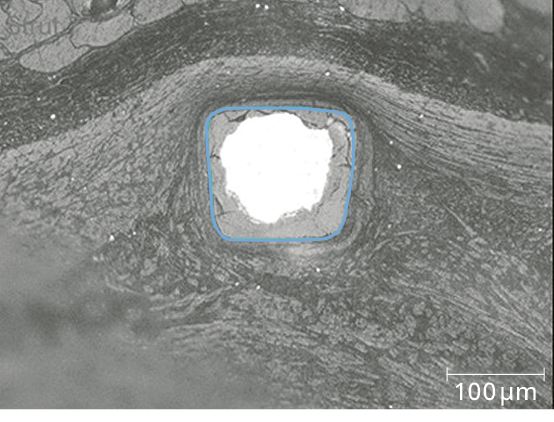
Uniform shape due to homogeneous strut resorption6
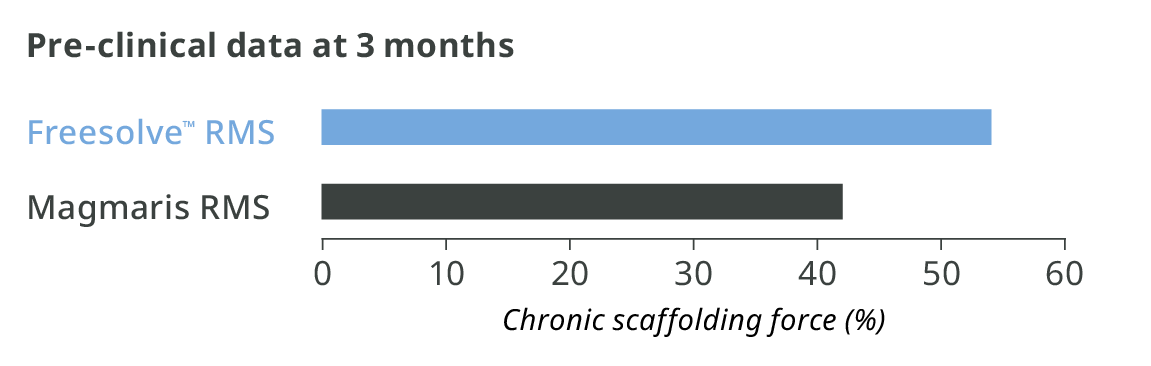
More than 3 months vessel support6,7
>99% of struts no longer visible at 12 months8
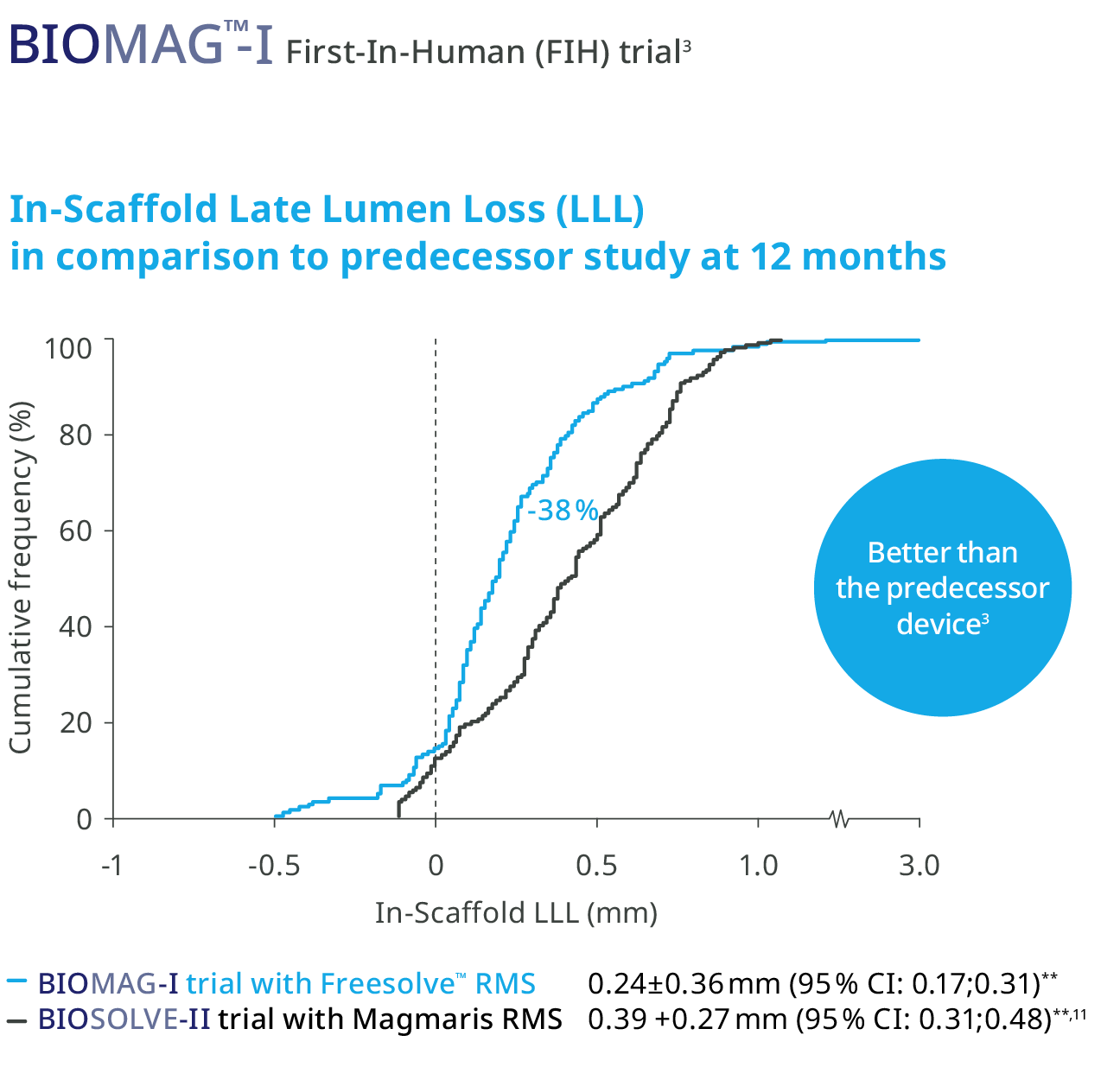
The in-scaffold Late Lumen Loss (LLL) for Freesolve™ RMS is on the level of a contemporary DES10
Freesolve™ RMS Median LLL: 0.19 mm3
Contemp. DES Median LLL: 0.18 mm10
36-month outcomes confirms, Freesolve RMS™ delivers on its promise12
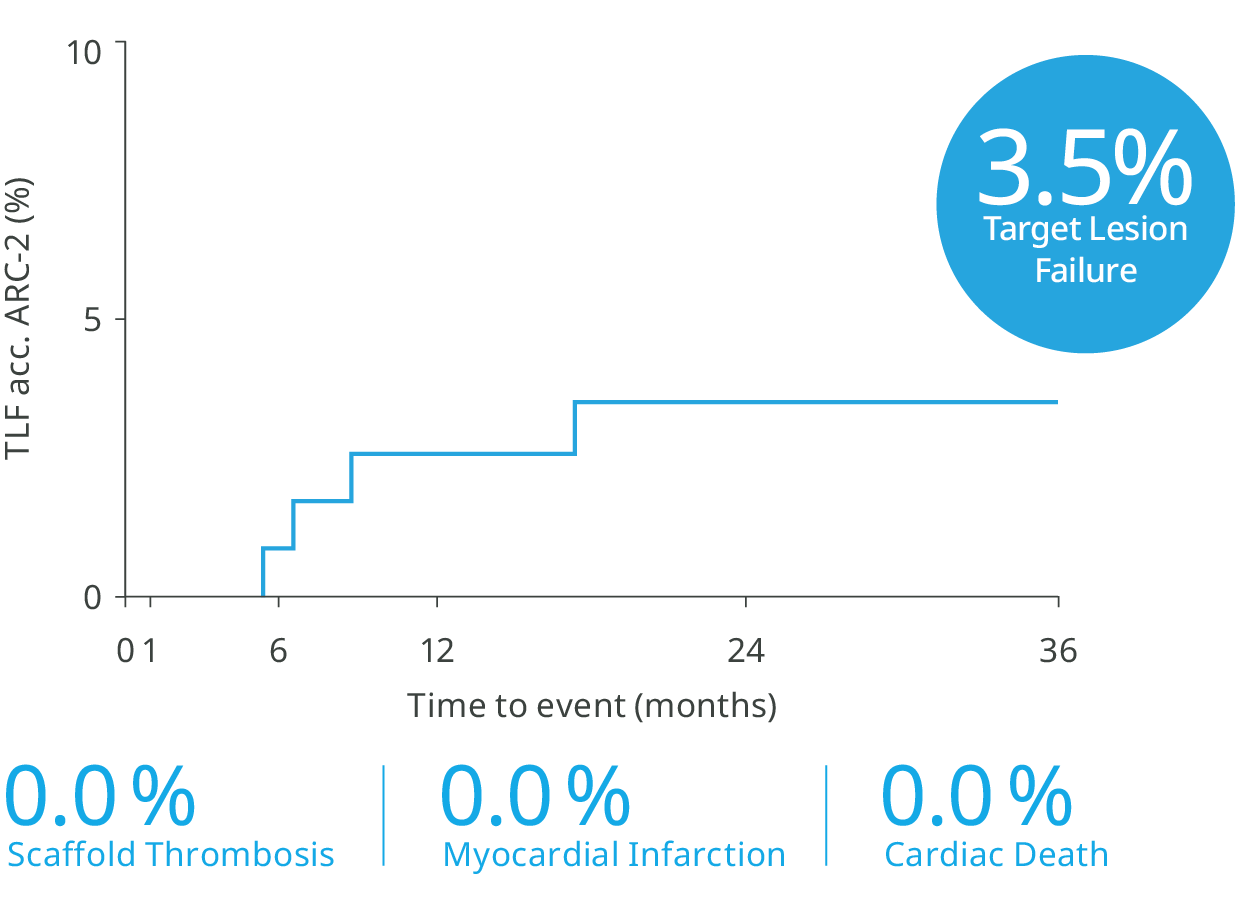
Clinical Highlights
In-Scaffold Late Lumen Loss (LLL) for Freesolve RMS is on the
level of a contemporary DES.
The prospective BIOMAG™-I clinical trial assesses the angiogrpahic,
clinical and safety performance of DREAMS 3G RMS of 116 patients
with single de novo lesions up to two coronary arteries.
Product Overview
| Scaffold ø (mm) (SD) |
Recommended ø (mm) (RVD) |
|---|---|
| 2.50 | 2.50 – 2.70 |
| 3.00 | 2.70 – 3.20 |
| 3.50 | 3.20 – 3.70 |
| 4.00 | 3.70 – 4.20 |
| Balloon diameter (mm) | ||||
|---|---|---|---|---|
| ø 2.50 | ø 3.00 | ø 3.50 | ||
| Nominal Pressure (NP) |
atm** ø (mm) |
10 2.52 |
10 3.04 |
10 3.54 |
| Rated Burst Pressure (RBP) |
atm** ø (mm) |
16 2.72 |
16 3.29 |
16 3.79 |
| *1 atm = 1.013 bar | ||||
| Scaffold ø (mm) |
Scaffold length (mm) |
||||
|---|---|---|---|---|---|
| 13 | 18 | 22 | 26 | 30 | |
| 2.50 | 443103 | 443104 | 443105 | - | - |
| 3.00 | 443108 | 443109 | 443110 | 482156 | 443111 |
| 3.50 | 443113 | 443114 | 443115 | 482157 | 443116 |
| 4.00 | 443118 | 443119 | 443120 | 482158 | 443121 |