Customer Support
Teleflex Medical Australia
Suite 3, Level 7, Building 3, Connect Corporate Centre 189 O'Riordan Street, Mascot NSW 2020 Customer Service: 1300-360-226 (Domestic calls only) Email Teleflex Medical Australia
Teleflex Medical New Zealand
300 Richmond Road
Grey Lynn
Auckland 1021
New Zealand
Customer Service: 0800 601 100 (Domestic calls only)
Email Teleflex Medical New Zealand

GuideLiner V3 Catheter
Guide extension catheter with half-pipe technology
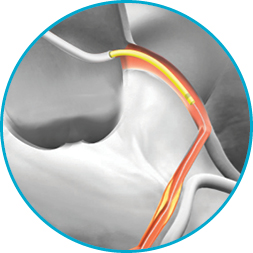
In 2009, the GuideLiner Catheter revolutionised the concept of guide extension, creating new possibilities in interventional cardiology. Now in its third generation, the GuideLiner V3 Catheter continues to build on a history of innovation and performance—one that's been demonstrated with more than half a million catheters in cath labs around the world.1
Designed to perform
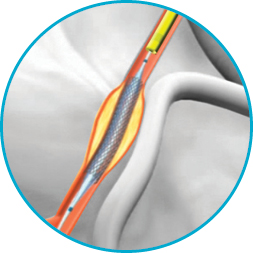
It's not just the proprietary half-pipe technology that sets the GuideLiner V3 Catheter apart. The coil-reinforced extension is specifically designed to enable dependable deep-seating for the delivery of interventional devices to distal locations.
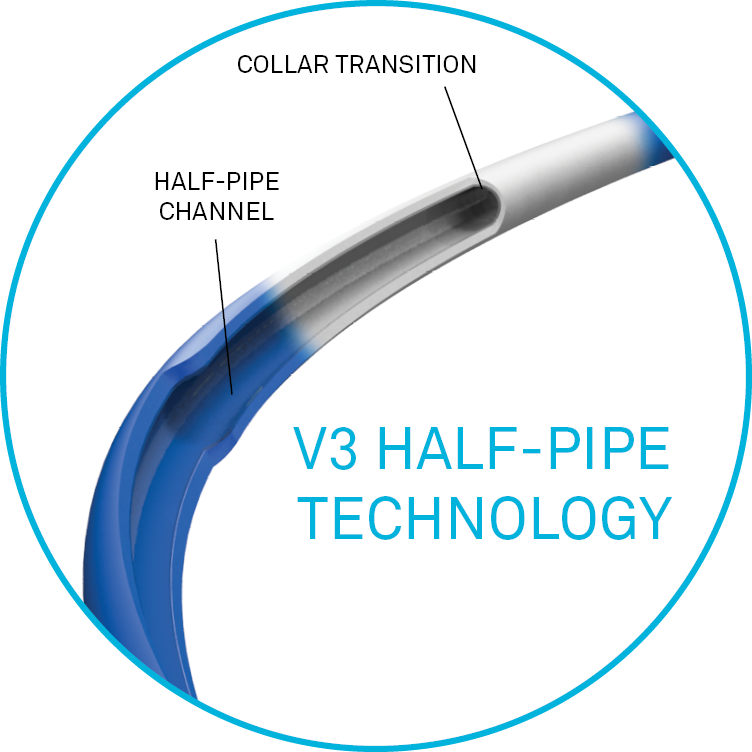
Half-pipe technology
The half‑pipe channel is designed to minimise device/collar interaction by directing and aligning devices through the collar transition, facilitating smooth device entry and seamless delivery.
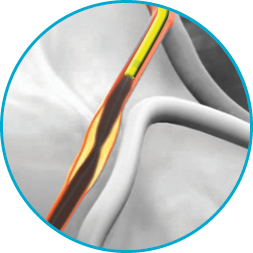
Tortuosity tested
From challenging lesions to impossibly acute angles, percutaneous coronary interventions have grown more complex. Since being introduced, GuideLiner Catheters have been recognised by interventionalists as essential tools for addressing difficult anatomies.
Available in five sizes: 5, 5.5, 6, 7 and 8 Fr.
References:
- Data on file.