Customer Support
Product Orders
(866) 246-6990 USA Product orders by FAX: (866) 804-9881 USA Email Customer Service Contact Us Form

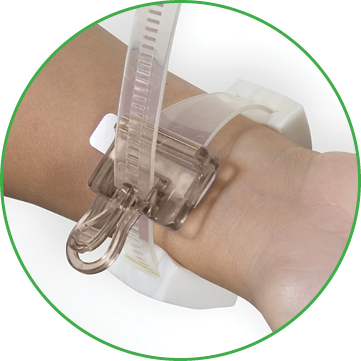
D-Stat® Rad-Band Topical Hemostat
Utilizing the power of thrombin
The D-Stat® Rad-Band Topical Hemostat contains the power of thrombin in a familiar D-Stat® Dry Topical Hemostat pad. The device includes an easy release clasp for single-operator pressure adjustments, and adjustable foam pads for additional patient comfort.
The D-Stat® Rad-Band is contraindicated in persons with known sensitivity to bovine-derived materials.
WARNING: SEVERE
BLEEDING AND THROMBOSIS COMPLICATIONS
- THROMBIN-JMI® can cause fatal severe bleeding or thrombosis. Thrombosis may
result from the development of antibodies against bovine thrombin. Bleeding may result from the
development of antibodies against factor V. These may cross-react with human factor V and lead to
its deficiency.
- Do not re-expose patients to THROMBIN-JMI® if there are known or suspected
antibodies to bovine thrombin and/or factor V.
- Monitor patients for abnormal coagulation laboratory values, bleeding, or thrombosis.
CAUTION: Federal (USA) law restricts this device to sale by or on the order of a physician.
Not all products are available in all regions. Please contact customer service to confirm availability in your region.
THROMBIN-JMI is a registered trademark of King Pharmaceuticals Research and Development, LLC.
Teleflex, the Teleflex logo and D-Stat are trademarks or registered trademarks of Teleflex Incorporated or its affiliates, in the U.S. and/or other countries. MC-004254 Rev 0
Revised: 04/2018.